1. Typical left-ventricular hypertrophy (tall precordial R waves in leads V2 to V5; see Chapter 5).
2. Deep, narrow Q waves in the leftward-oriented leads (aVL and V6).
3. Left atrial enlargement (increased terminal P-wave negativity in lead V1; see Chapter 5).
Amyloidosis
An abnormal protein called amyloid is deposited in the heart during various disease processes. Its accumulation causes cardiac amyloidosis, which eventually may produce sufficient cardiomyopathy to cause heart failure. Amyloidosis may be suspected when the following combination of ECG changes appear1:
1. Low voltage of all waveforms in the limb leads.
2. Marked left-axis deviation typical of left-anterior fascicular block.
3. Pseudo infarct changes.
4. A prolonged atrioventricular (AV) conduction time.
Characteristics 1 and 3 are apparent in an elderly patient with severe heart failure but no history of ischemic heart disease (Fig. 13.2). Note the extremely low voltage in both limb and precordial leads and pseudo infarct changes; Q waves are typically seen with both inferior and anterior infarcts.

A small, fluid-filled space called the pericardial sac separates the heart from the other structures in the thorax. The sac is lined by two layers of connective tissue referred to as the pericardium. The innermost of these two layers (visceral pericardium) adheres to the myocardium, and the outer layer (parietal pericardium) encloses the pericardial fluid. These two layers of tissue can become inflamed for many reasons (pericarditis). The inflammation usually resolves after an acute phase but may progress to a chronic phase. The acute phase may be complicated by the collection of excess pericardial fluid, a condition termed pericardial effusion. Chronic persistence of the inflammatory process may result in thickening of the pericardial tissues and is called constrictive pericarditis.
Acute Pericarditis
Typically, acute pericarditis persists for 3 or 4 weeks, and the ECG changes it produces evolve through two stages. The recordings in Figure 13.3 are from a patient presenting with the chest pain of acute pericarditis (see Fig. 13.3A) and returning to the clinic 1 month later (see Fig. 13.3B). The characteristic ECG abnormality during the earliest stage of acute pericarditis is elevation of the ST segments in many leads, accompanied by upright T waves (see Fig. 13.3A).2 Depression of the PR segment was also present in half of a series of consecutive patients with acute pericarditis.3 When the ST segments return to the isoelectric level, the ECG may appear normal (see Fig. 13.3B).
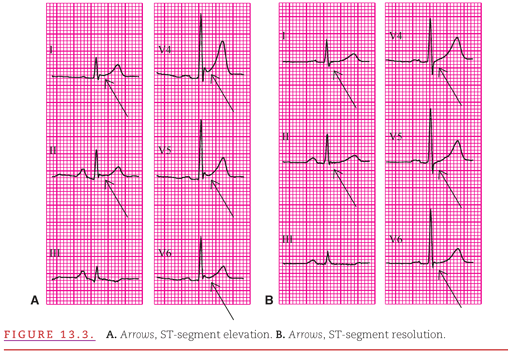
The ST-segment elevation in the first stage of acute pericarditis occurs because the inflammation also involves the immediately adjacent epicardial layer of myocardium, producing an epicardial “injury current” similar to transmural myocardial ischemia discussed in Chapter 11. When the epicardial injury is caused by insufficient myocardial perfusion (i.e. ischemia), the ST-segment elevation is restricted to the ECG leads that overlie the myocardial region supplied by the obstructed coronary artery. Because pericarditis usually involves the entire epicardium, there is ST-segment elevation in all of the standard leads that are positive leftward and anteriorly and with ST depression in lead aVR. However, differentiation between acute pericarditis and acute myocardial ischemia becomes difficult when the pericarditis is localized, creating ST-segment elevation in only a few leads.
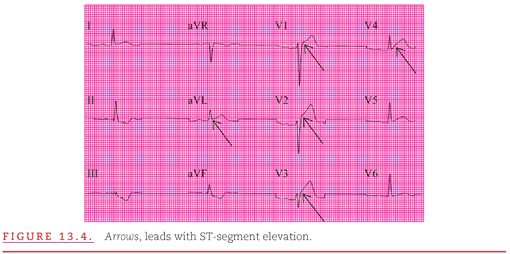
Figure 13.4 illustrates such an example with a 12-lead ECG from a woman with breast carcinoma and acute chest pain. In both acute pericarditis and acute myocardial infarction, the patient may present with precordial pain, and additional clinical evaluation is necessary to reach the appropriate diagnosis. Serial ECG recordings are useful because the acute epicardial injury current of decreased coronary flow is transient and resolves when the region is either infarcted or reperfused, but the changes of pericarditis persist until the inflammation resolves.
Acute pericarditis also often resembles the normal variant termed “early repolarization” discussed in Chapter 3. Figure 13.5 presents a typical example of a routine 12-lead ECG recorded from a healthy young man that could represent either the early repolarization or the first stage of acute pericarditis.

A factor on this example that favors a diagnosis of acute pericarditis is the widespread ST elevation, but another factor that favors early repolarization is the increased T-wave amplitude in several leads (see Table 12.1). This emphasizes the point that it is often not possible to distinguish on the ECG between these very different conditions.
Pericardial Effusion and Chronic Constriction
Small and even moderate amounts of pericardial effusion or constriction may have little or no effect on the ECG. However, a generalized decrease in all of the ECG waveform amplitudes (low voltage) occurs if significant pericardial effusion or thickening develops. This probably occurs because the cardiac impulses are dampened by the pericardial fluid or fibrotic thickening. Because both of these pathologic conditions have similar effects on the cardiac electrical activity and its transmission to the body surface, they are considered together. A triad of ECG changes that is virtually diagnostic of pericardial effusion or constriction is presented below:
1. Low voltage.
2. Widespread ST-segment elevation.
3. Total electrical alternans.
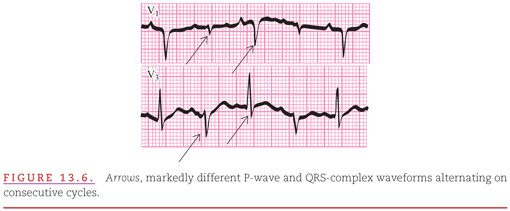
These changes are observed in ECG leads V1 and V3 recorded from a patient with carcinoma of the lung and malignant pericardial effusion (Fig. 13.6). Total electrical alternans refers to the alternating high and low voltages of all ECG waveforms between cardiac cycles within a given lead.4,5
In addition to these ECG effects, chronic constrictive pericarditis may be accompanied by the T-wave inversion that defines the second stage of acute pericarditis.6 The depth of inversion of the T waves has been reported to correlate with the degree of pericardial adherence to the myocardium. This may be clinically important because surgical “stripping” of the thickened pericardium is more difficult when it is adhered tightly to the myocardium.
When a pulmonary abnormality creates an increased resistance to blood flow from the right side of the heart, a condition of systolic or pressure overload develops (see Chapter 5). This condition has been termed cor pulmonale, and it can occur either acutely or chronically. The most common cause of acute cor pulmonale is pulmonary embolism. Chronic cor pulmonale may be produced by the pulmonary congestion that occurs with left-ventricular failure or by the pulmonary hypertension that develops either as a primary disease or as a secondary disease to chronic obstructive pulmonary disease. Right-atrial enlargement commonly occurs with acute and chronic cor pulmonale. In the acute condition, there is right-ventricular dilation, whereas in the chronic condition there is right-ventricular hypertrophy (RVH) at first, then RV dilatation. Because chronic RVH is discussed in detail in Chapter 5, only acute cor pulmonale is included here.
Chronic obstructive pulmonary disease is often characterized by emphysema, in which the lungs become overinflated. This produces anatomic changes that affect the ECG in unique ways. The ECG changes of pulmonary emphysema may occur alone or in combination with the changes of RVH because emphysema may or may not be accompanied by the obstruction of the airways. When CO2 is unable to escape through the tracheal–bronchial system, the condition of hyperventilation occurs. The resultant hypercapnia (elevated blood CO2 levels) and respiratory acidosis cause the pulmonary arterial constriction leading to the compensatory RVH that is termed chronic cor pulmonale.
Acute Cor Pulmonale: Pulmonary Embolism
Acute cor pulmonale in the absence of evidence of the changes of RVH owing to chronic cor pulmonale is most commonly seen in pulmonary embolism. Acute cor pulmonale can occur in the presence or absence of chronic changes of RVH. The ECG changes considered herein are those in the absence of RVH. The RV distortion produced by an acute outflow obstruction such as pulmonary embolism causes delayed conduction through the right bundle and/or the RV myocardium, resulting in the ECG pattern of incomplete or even complete right-bundle-branch block (RBBB; see Chapter 6). The subtraction of the RV contribution from the initial portion of the QRS complex shifts the waveforms away from both the inferior (limb leads) and anterior (chest leads), mimicking both inferior and anterior infarcts. This shift to the QRS beyond the completion of LV activation produces unopposed terminal rightward and anterior forces.7 The rightward direction is represented primarily by an S wave in lead I and the anterior direction by an R′ wave in V1. Figure 13.7 presents recordings from before (A) and after (B) sudden dyspnea in an elderly man who had received prostate surgery.

In the precordial leads, elevated ST segments and inverted T waves are sometimes seen over the right ventricle, whereas S waves may become more prominent over the left ventricle. The typical changes of RBBB may be apparent in lead V1 (a 12-lead from an elderly woman with a massive pulmonary embolism who exhibits the typical changes of RBBB is illustrated in Fig. 13.8). All of the ECG changes of the acute cor pulmonale produced by a large pulmonary embolus are seen in Figure 13.8. The RBBB is complete with a QRS duration of 120 milliseconds. In addition, there are the repolarization changes of T-wave inversion both in leads III and aVF and in leads V1 through V4.

Pulmonary Emphysema
The five most typical findings in emphysema have been grouped together as follows8:
1. Tall P waves in leads II, III, and aVF.
2. Exaggerated atrial repolarization waves producing ≥0.10-mV PR and ST-segment depression in leads II, III, and aVF.
3. Rightward shift of the axis of the QRS complex in the frontal plane.
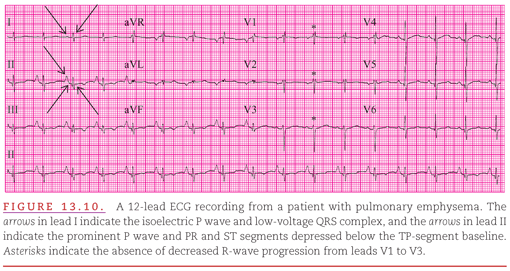
4. Decreased progression of the R-wave amplitudes in the precordial leads.
5. Low voltage of the QRS complexes, especially in the left precordial leads.
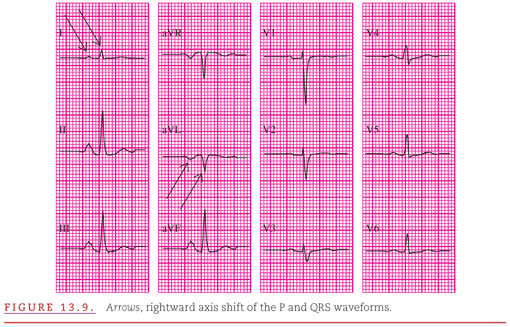
Figure 13.9 presents a typical example of pulmonary emphysema with all five of these characteristics. Rightward shifts of both the P waves and QRS complexes (negative in lead aVL and only slightly positive in lead I) and a low voltage in the leftward (V4 to V6) precordial leads are illustrated (Fig. 13.9). Note the prominent P waves in II, III, and aVF followed by PR- and ST-segment depression below the TP baseline.
These ECG changes are produced because the hyperexpanded emphysematous lungs compress the heart, lower the diaphragms, and increase the space between the heart and the recording electrodes.
The QRS axis in the frontal plane is occasionally indeterminate (Fig. 13.10).9 This occurs because pulmonary emphysema directs the QRS complex posteriorly so that minimal upward or downward deviation swings the frontal plane axis of the complex from +90 degrees to −90 degrees. Figure 13.10 also illustrates criteria 1, 2, 3, and 4 in the list given above.
Selvester and Rubin10 have developed quantitative ECG criteria for both definite and possible emphysema (Table 13.1).

These criteria achieve approximately 65% sensitivity for the diagnosis of emphysema and 95% specificity for the exclusion of emphysema in normal control subjects and in patients with congenital heart disease or myocardial infarction.9 This good performance relative to that of other systems is most likely the result of combining quantitative criteria for the frontal plane P-wave axis with criteria for both the frontal and transverse plane amplitudes of the QRS complex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


