
The mechanism by which a tachyarrhythmia is perpetuated determines the treatment required for its management. Accelerated automaticity can best be treated by eliminating the cause of the acceleration rather than by treating the acceleration itself. When the accelerated automaticity originates from the sinus node, the cause is increased sympathetic nervous activity resulting from systemic conditions such as exertion, anxiety, fever, decreased cardiac output, or thyrotoxicosis. When the accelerated automaticity originates from another location, the most common causes are ischemia and digitalis toxicity. Therefore, accelerated sinus automaticity is treated by removing the responsible systemic condition, and accelerated nonsinus automaticity, termed “ectopic,” is treated by removing the responsible condition.
PROBLEMS OF IMPULSE CONDUCTION: BLOCK
The term block is used to refer to the situation in which conduction is slowed or fails to occur at all (e.g., AV block; see Chapter 22) or bundle-branch block (see Chapter 6). Cardiac impulses can be either partly blocked, causing conduction delay (e.g., a prolonged PR interval), or totally blocked, causing conduction failure (e.g., complete AV block). With a partial block of impulses, there is no change in the rate of the affected site, but with a total block of impulses, a bradyarrhythmia is produced in the affected site. Either partial or total block can occur at any site within the pacemaking and conduction system (Table 14.1).
PROBLEMS OF IMPULSE CONDUCTION: REENTRY
Although conduction abnormalities sufficient to produce block can occur only within the pacemaking and conduction systems, uneven or inhomogeneous conduction can occur in any part of the heart. This inhomogeneous spread of electrical impulses can result in reentry of an impulse into an area that has just previously been depolarized and repolarized.1 Reentry produces a circular movement of the impulse, which continues as long as the impulse encounters receptive cells, resulting in a single premature beat, multiple premature beats, a nonsustained tachyarrhythmia, or even a sustained tachyarrhythmia. There are three prerequisites to the development of reentry:
1. An available circuit.
2. A difference in the refractory periods of the two pathways (limbs) in the circuit.
3. Conduction that is sufficiently slow somewhere in the circuit to allow the remainder of the circuit to recover its responsiveness by the time the impulse returns.
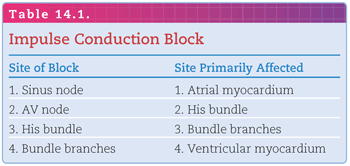
In Figure 14.2, the diagrams represent three different situations regarding the homogeneity of pathway receptiveness to impulse conduction: (A) Both limbs of the pathway are receptive—the left and right limbs have completed the recovery process and are receptive to the entering impulse; (B) both limbs of the pathway are refractory—the left and right limbs are still refractory (because of the persisting depolarized state) to being reactivated by the entering impulse; (C1) one limb of the pathway is receptive and the other is refractory; (C2) the left limb of the pathway is refractory and the right limb is receptive. By the time the impulse reaches the distal end of the left limb (by traveling down the right limb), it is able to reenter because repolarization has been completed. The impulse continues to cycle within the re-entry circuit as long as it encounters receptive cells, thus producing a reentrant tachyarrhythmia.
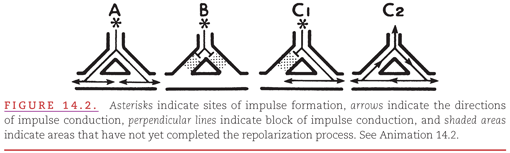

An example of the development of a “re-entry circuit” in the presence of an accessory AV conduction pathway is presented in Figure 14.3. During sinus rhythm (see Fig. 14.3A), both the AV node and the bundle of Kent have had time to recover from their previous activation. The premature atrial beat in Figure 14.3B encounters persisting refractoriness in the nearby bundle of Kent but encounters receptiveness in the more distant AV node, a situation analogous to that shown in Figure 14.2C1. This leads to the development of a re-entry circuit (see Fig. 14.3C) analogous to that in Figure 14.2C2. Re-entry circuits vary in size from a local area of myocardial fibers (see Fig. 14.2C2) to atrial and ventricular chambers (see Fig. 14.3C).

The term micro–re-entry describes the mechanism that occurs when a re-entry circuit is too small for its activation to be represented by a waveform on the surface ECG. The impulses formed within the re-entry circuit spread through the surrounding myocardium just as they would spread from an automatic or pacemaking site. The P waves and QRS complexes on the ECG are produced by this passive spread of activation through the atria and ventricles. Micro–re-entry commonly occurs in the AV node (see Chapter 18) and in the ventricles (see Chapter 19).
The term macro–re-entry describes the mechanism that occurs when a re-entry circuit is large enough for its own activation to be represented on the surface ECG (see Fig. 14.3). Cycling of the activating impulse through the right atrium (see Fig. 14.3C) is represented by a portion of the P wave, with the remainder of the P wave produced by spread of the impulse through the uninvolved left atrium. Cycling of the impulse through the right ventricle (see Fig. 14.3C) is represented by a portion of the QRS complex, with the remainder of the QRS complex produced by spread of the impulse through the uninvolved left ventricle.
There are also forms of macro–re-entry in which the re-entry circuit is entirely within either the atrial or ventricular myocardium. When this occurs, sawtooth-like or undulating ECG waveforms replace discrete P waves (see Chapter 17) or QRS complexes (see Chapter 19).
In attempting to treat reentry, it is important to understand its mechanism. For any re-entry circuit to perpetuate itself, the advancing head of the recycling impulse must not catch up with the refractory tail (Fig. 14.4A). Thus, there must always be a gap of nonrefractory cells between the head and the tail of the recycling impulse. A sustained reentrant tachyarrhythmia can be terminated by:
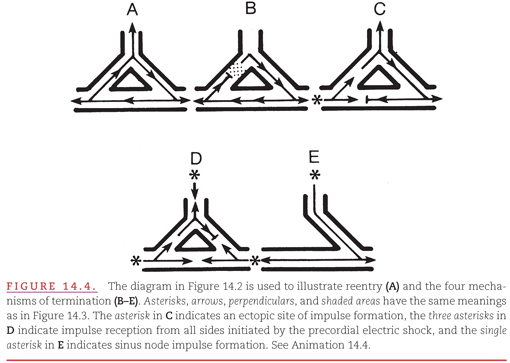

1. Administering drugs that accelerate conduction of the impulse in the re-entry circuit so that it encounters an area that has not yet recovered (see Fig. 14.4B). Termination also results if a drug prolongs the recovery time.
2. Introducing an impulse from an artificial pacemaker that depolarizes (captures) part of the re-entry circuit, thereby rendering it nonreceptive to the returning impulse (see Fig. 14.4C).
3. Introducing a precordial electrical shock, termed cardioversion, that captures all receptive parts of the heart, including those in the re-entry circuit, rendering the circuit nonreceptive to the returning impulse (see Fig. 14.4D).
4. Performing surgical or catheter ablation of one limb of the tissue required for the re-entry circuit. For example, ablation of the accessory AV pathway in a patient with ventricular preexcitation (see Fig. 14.4E).
CLINICAL METHODS FOR DETECTING ARRHYTHMIAS
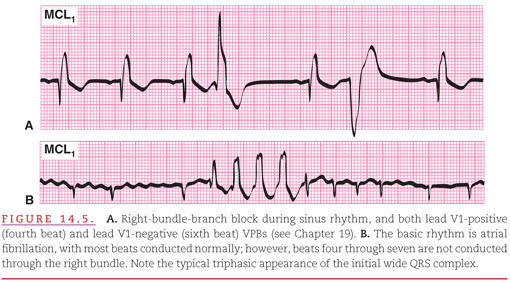
The introduction of coronary care units (CCUs) in the early 1960s stimulated rapid advances in the diagnosis and treatment of cardiac arrhythmias. In the CCU, patients who have either arrhythmias, or a high risk of developing arrhythmias because of conditions such as acute myocardial infarction, are continuously monitored (see Chapter 2). A modified chest lead V1 (MCL1) is commonly used as the display lead because it provides both a good view of atrial activity and of differentiation between right and left ventricular activity (Fig. 14.5).2 Often, however, multiple leads are displayed on both bedside and central surveillance monitors to provide multiple views of the cardiac electrical activity to facilitate rapid and accurate rhythm interpretation.
A method for continuous ECG monitoring of ambulatory patients in their own environment was developed in the 1960s by Holter3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


