The comparative efficacy and safety of angiotensin-converting enzyme inhibitors (ACEIs) with other agents in patients ≥65 years of age with cardiovascular diseases or at-risk are unknown. Electronic databases were systematically searched to identify all randomized controlled trials that compared ACEIs with control (placebo or active) and reported long-term cardiovascular outcomes. We required the mean age of patients in the studies to be ≥65 years. Random-effects model was used to pool study results. Sixteen trials with 104,321 patients and a mean follow-up of 2.9 years were included. Compared with placebo, ACEIs significantly reduced all outcomes except stroke. Compared with active controls, ACEIs had similar effect on all-cause mortality (relative risk [RR] 0.99, 95% confidence interval [CI] 0.95 to 1.03), cardiovascular mortality (RR 0.99, 95% CI 0.93 to 1.04), heart failure (RR 0.97, 95% CI 0.91 to 1.03), myocardial infarction (RR 0.94, 95% CI 0.88 to 1.00), and stroke (RR 1.07, 95% CI 0.99 to 1.15). ACEIs were associated with an increased risk of angioedema (RR 2.79, 95% CI 1.05 to 7.42), whereas risk for hypotension and renal insufficiency was similar compared with active controls. Meta-regression analysis showed that the effect of ACEIs on outcomes remained consistent with age increasing ≥65 years. Sensitivity analysis excluding trials comparing ACEIs with angiotensin receptor blockers and heart failure trials yielded similar results, except for reduction in myocardial infarction. In conclusion, the efficacy of ACEIs was similar to active controls for mortality outcomes. Compared with placebo, there was evidence for reduction in cardiovascular outcomes; however, ACEIs failed to prevent stroke and increased the risk of angioedema, hypotension, and renal failure.
In the United States, population aged ≥65 years is projected to nearly double to 84 million by 2050. With a rapidly aging population, the prevalence of cardiovascular diseases such as hypertension, heart failure, and coronary artery disease is expected to increase in parallel. By blocking the renin-angiotensin-aldosterone system, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) decrease blood pressure, reduce afterload, and prevent ventricular remodeling. However, the comparative effectiveness of ACEIs in patients ≥65 years and cardiovascular diseases is not well studied. Patients ≥65 years have an increased burden of co-morbidities and therefore are often exposed to polypharmacy, which may potentiate the risk of adverse effects. Despite these risks, there is a paucity of literature on adverse effects of ACEIs, particularly in patients ≥65 years of age. We conducted a comprehensive meta-analysis to study the long-term efficacy and safety of ACEIs in patients ≥65 years and who either had cardiovascular diseases or were at high risk for cardiovascular diseases.
Methods
We performed a systematic search, without language restriction, using MEDLINE, Cochrane library, ClinicalTrials.gov , Embase, and Scopus databases from January 1, 1987, to March 1, 2016, for randomized controlled trials (RCTs) that compared ACEIs with placebo/active control and reported mortality and other cardiovascular outcomes. The search keywords included the following MeSH terms: (angiotensin-converting enzyme inhibitors OR ACEI OR benazepril OR captopril OR enalapril OR fosinopril OR lisinopril OR perindopril OR quinapril OR ramipril OR trandolapril) AND (randomized controlled trial or clinical trial) AND (mortality OR death OR cardiovascular events). Furthermore, we performed manual searches through the reference lists of studies, reviews, and meta-analyses on this topic to identify pertinent studies missed by the search strategy. When there were multiple reports from the same trial, we used the most complete and relevant reported data.
Studies were included if they met the following inclusion criteria: (1) RCTs comparing ACEIs with placebo or active control; (2) trials providing data on all-cause mortality and other cardiovascular outcomes; and (3) mean age of the patients in the studies should be ≥65 years. Exclusion criteria were the following: (1) ACEIs not used as the first-line therapy; (2) ACEIs used in combination with other agents; and (3) study reported only noncardiovascular or renal outcomes.
Two reviewers (CB and MA) independently and in duplicate performed the literature search, reviewed the originally identified titles and abstracts, and selected studies for pooled analysis based on the inclusion and exclusion criteria. Any divergence was resolved with consensus. Quality of the included studies and assessment of trial bias risk were assessed for the domains suggested by the Cochrane Collaboration, specifically emphasizing sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. The efficacy outcomes evaluated were all-cause mortality, cardiovascular mortality, myocardial infarction (MI), stroke, and hospitalization for heart failure, whereas angioedema, hypotension, and renal insufficiency were assessed as safety outcomes.
The statistical analysis was performed in line with recommendations from the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We analyzed the efficacy and safety of ACEIs separately by placebo and active controls. Considering that the heterogeneity of the included trials might influence the treatment effects, we used the random-effects model to pool studies. Analysis was performed on an intention-to-treat basis. Heterogeneity was assessed using Higgins and Thompson’s I 2 statistic with I 2 values of <25%, 25% to 75%, and >75% corresponding to low, moderate, and high levels of heterogeneity, respectively. We performed sensitivity analysis by excluding trials comparing ACEIs and ARBs and heart failure trials. Publication bias was estimated visually by funnel plots and Egger’s regression test. We also performed metaregression analysis to evaluate any association of age with ACEIs and outcomes. A 2-tailed p value <0.05 was considered statistically significant for all the analyses. All statistical analyses were performed using Stata 11 (Stata Corp., College Station, Texas) and RevMan v5.02 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014).
Results
Overall, 16 randomized trials fulfilled all selection criteria for this meta-analysis ( Supplementary Figure 1 available online only). The trials were either placebo controlled or active comparator controlled. The Pilot Hypertension in the Very Elderly Trial (HYVET) had both placebo and active controlled arms. We excluded the combination arms (ACEIs plus ARBs) of the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) and the Valsartan in Acute Myocardial Infarction Trial. Six ACEIs (captopril, enalapril, lisinopril, perindopril, ramipril, and trandolapril) were included in this meta-analysis. In total, 104,321 patients were randomized to either ACEIs or controls (placebo or active comparator). The average follow-up was 2.9 years (range 0.9 to 4.7 years), with a total follow-up across all trials of 302,531 patient-years. We included 1-year follow-up results from the Perindopril in Elderly People With Chronic Heart Failure trial as a substantial number of patients ceased blinded treatment after 1 year. The characteristics of the included trials are listed in Table 1 . The overall mean age of patients in ACEI and control arms was 69.7 years. The baseline characteristics of patients with mean blood pressure at baseline and at the end of the studies are listed in Table 1 . Five trials (including Japan Multicenter Investigation for Cardiovascular Diseases-B [JMIC-B] trial with coronary artery disease and hypertension) involved patients with hypertension, 3 trials involved patients with diabetes mellitus and high cardiovascular risk, and the remaining trials involved patients with coronary artery disease, heart failure, or both. Most of the included studies were of low-bias risk, except for 4 studies that were high-bias risk mainly for being open-label studies ( Table 2 ).
| Trials | Patients | Age (years) | Male | HTN | HF | DM | Baseline BP, mm Hg | BP end follow- up, mm Hg | Follow- up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACEI | Control | ACEI | Control | ACEI | Control | ACEI | Control | ACEI | Control | ACEI | Control | ACEI | Control | ACEI | Control | ||
| AIRE , 1993 | 1,004 | 982 | 65 | 65 | 73% | 74% | 29% | 27% | 8% | 8% | 12% | 12% | NR | NR | NR | NR | 15 months |
| ALLHAT , 2002 | 9,054 | 24,303 | 67 | 67 | 54% | 53% | 90% | 90% | 0 | 0 | 36% | 36% | 146/84 | 146/84 | 136/75 | 134/75 | 4.9 years |
| ANBP2 , 2003 | 3,044 | 3,039 | 72 | 72 | 50% | 48% | 100% | 100% | NR | NR | 8% | 7% | 167/91 | 168/91 | 141/79 | 142/79 | 4.1 years |
| CONSENSUS , 1987 | 127 | 126 | 71 | 70 | 70% | 71% | 24% | 19% | 100% | 100% | 24% | 21% | 118/74 | 121/76 | NR | NR | 1 year |
| DIABHYCAR , 2004 | 2,443 | 2,469 | 65 | 65 | 70% | 70% | 56% | 55% | 0 | 0 | 100% | 100% | 146/82 | 145/82 | 142/80 | 143/80 | 4 years |
| ELITE , 1997 | 370 | 352 | 73 | 74 | 67% | 65% | 57% | 57% | 100% | 100% | 24% | 27% | 137/79 | 137/79 | NR | NR | 48 weeks |
| ELITE II , 2003 | 1,574 | 1,578 | 72 | 71 | 69% | 70% | 50% | 48% | 100% | 100% | 24% | 24% | 134/78 | 134/78 | NR | NR | 555 days |
| HOPE , 2000 | 4,645 | 4,652 | 66 | 66 | 73% | 74% | 48% | 46% | 0 | 0 | 39% | 38% | 139/79 | 139/79 | 136/76 | 139/77 | 5 years |
| JMIC-B , 2004 | 822 | 828 | 64 | 65 | 70% | 68% | 100% | 100% | NR | NR | 21% | 24% | 145/82 | 147/82 | 138/79 | 136/77 | 3 years |
| ONTARGET , 2008 | 8,576 | 8,542 | 66 | 66 | 73% | 74% | 69% | 69% | 0 | 0 | 37% | 38% | 142/82 | 142/82 | NR | NR | 56 months |
| OPTIMAAL , 2002 | 2,733 | 2,744 | 67 | 68 | 71% | 72% | 36% | 36% | 6.5% | 5.9% | 17% | 18% | 123/71 | 123/72 | NR | NR | 2.7 years |
| PEP-CHF , 2006 | 424 | 426 | 75 | 75 | 46% | 43% | 79% | 79% | 100% | 100% | 21% | 20% | 138/80 | 140/80 | 135 | 138 | 2.1 years |
| Pilot HYVET , 2003 | 431 | 852 | 84 | 84 | 36% | 37% | 100% | 100% | NR | NR | NR | NR | 182/100 | 182/100 | 151/84 | 163/90 | 13 months |
| STOP 2 , 1999 | 2,205 | 4,409 | 76 | 76 | 34% | 33% | 100% | 100% | 2% | 2% | 11% | 11% | 194/98 | 194/98 | 159/81 | 159/80 | 4 years |
| TRACE , 1995 | 876 | 873 | 68 | 67 | 72% | 71% | 23% | 23% | 21% | 23% | 13% | 14% | 122/76 | 120/75 | NR | NR | 24-50 months |
| VALIANT , 2006 | 4,909 | 4,909 | 65 | 65 | 69% | 69% | 55% | 56% | 15% | 16% | 23% | 23% | 123/72 | 123/72 | NR | NR | 25 months |
| Trial | Patient characteristics | ACEIs | Controls | Primary outcome | Blinded outcome assessment | Generation of treatment assignment | Follow up % | Study Quality |
|---|---|---|---|---|---|---|---|---|
| AIRE | CAD, HF | Ramipril | Placebo | All-cause mortality | Double blinded | Central randomization system | NR | Low-bias risk |
| ALLHAT | HTN | Lisinopril | Chlorthalidone/Amlodipine | Fatal coronary heart disease or non-fatal MI | Double blinded | Central randomization system | 97 | Low-bias risk |
| ANBP2 | HTN, ≥65 years | ACEIs | Diuretic | All-cause mortality or cardiovascular events | Open label with blinded end points | Central randomization system | 97 | High-bias risk |
| CONSENSUS | HF | Enalapril | Placebo | All-cause mortality | Double blinded | Computer generated | NR | Low-bias risk |
| DIABHYCAR | DM II, proteinuria | Ramipril | Placebo | Cardiovascular death, non-fatal MI, stroke, HF, end-stage renal failure | Double blinded | Centralized randomization system | 83 | Low-bias risk |
| ELITE | HF | Captopril | Losartan | Measure of renal dysfunction | Double blinded | NR | NR | Low-bias risk |
| ELITE II | HF, ≥60 years | Captopril | Losartan | All-cause mortality | Double blinded | NR | 89 | Low-bias risk |
| HOPE | DM, high risk patients | Ramipril | Placebo | MI, stroke, cardiovascular mortality | Double blinded | NR | NR | Low-bias risk |
| JMIC-B | CAD, HTN | ACEIs | Nifedipine | Cardiac/sudden death, MI, angina, HF, arrhythmia, coronary interventions | Open label with blinded end points | Computer generated | 79 | High-bias risk |
| ONTARGET | DM, high risk patients | Ramipril | Telmisartan | Cardiovascular death, MI, stroke, hospitalization for HF | Double blinded | Central randomization system | 100 | Low-bias risk |
| OPTIMAAL | CAD, HF | Captopril | Losartan | All-cause mortality | Double blinded | Central randomization system | 100 | Low-bias risk |
| PEP-CHF | HF, ≥70 years | Perindopril | Placebo | All-cause mortality, unplanned HF related hospitalization | Double blinded | Computer generated | 100 | Low-bias risk |
| Pilot HYVET | HTN, ≥80 years | Lisinopril | Diuretic/placebo | Stroke events, total mortality, cardiovascular mortality | Open label | Computer generated | 98 | High-bias risk |
| STOP 2 | HTN, ≥70 years | Lisinopril/enalapril | Beta-blockers/diuretics/CCBs | Cardiovascular mortality | Open label with blinded end points | NR | 100 | High-bias risk |
| TRACE | CAD, HF | Trandolapril | Placebo | All-cause mortality | Double blinded | Computer generated | 63 | Low-bias risk |
| VALIANT | CAD, HF | Captopril | Valsartan | All-cause mortality | Double blinded | Interactive voice-response system | 99 | Low-bias risk |
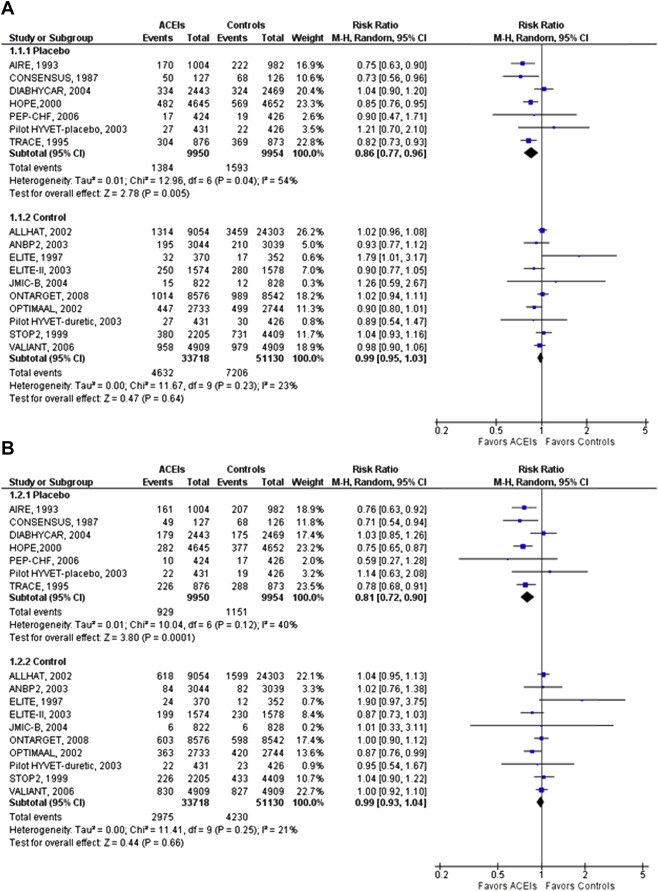
ACEIs significantly reduced all-cause mortality compared with placebo (relative risk [RR] 0.86, 95% confidence interval [CI] 0.77 to 0.96). However, compared with active control, ACEIs had similar effect on all-cause mortality (RR 0.99, 95% CI 0.95 to 1.03, p = 0.02 for interaction; Figure 1 ). Similarly, the cardiovascular mortality benefit was demonstrated when ACEIs were compared with placebo (0.81, 95% CI 0.72 to 0.90) but not when compared with active controls (0.99, 95% CI 0.93 to 1.04, p = 0.01 for interaction; Figure 1 ). There was moderate heterogeneity and no evidence for publication bias.
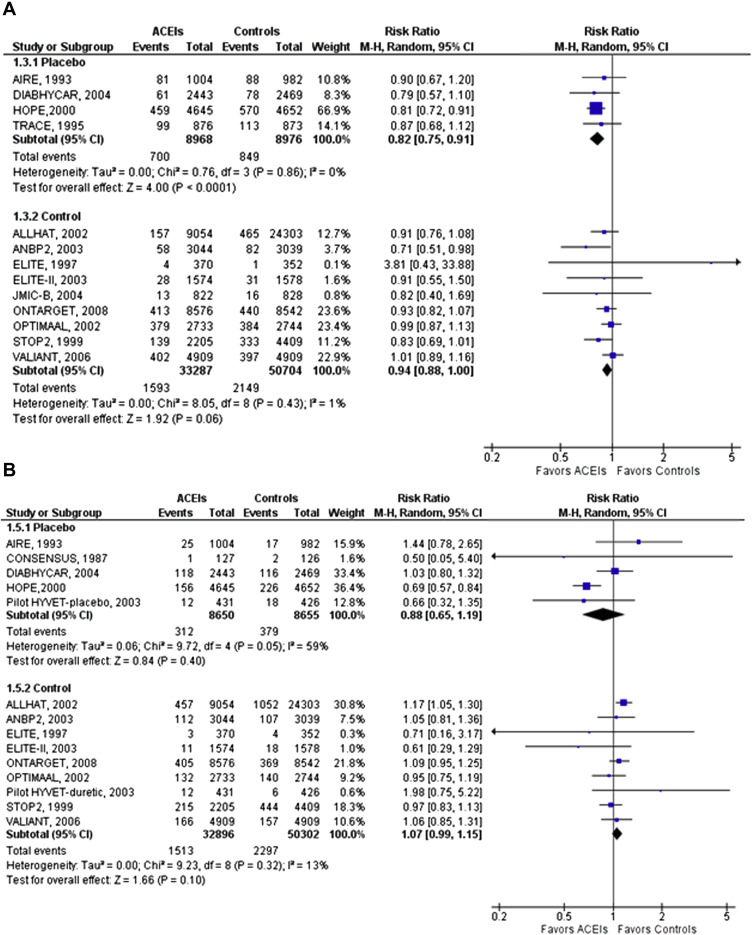
Compared with placebo, ACEIs significantly reduced MI (RR 0.82, 95% CI 0.75 to 0.91) and heart failure (RR 0.76, 95% CI 0.70 to 0.83). However, no reduction in stroke was found (RR 0.88, 95% CI 0.65 to 1.19; Figure 2 ). Compared with active control, ACEIs were not associated with reduction in MI (RR 0.94, 95% CI 0.88 to 1.00), heart failure (RR 0.97, 95% CI 0.91 to 1.03), and stroke (RR 1.07, 95% CI 0.99 to 1.15; Figure 2 and Supplementary Figure 2 available online only). There was low to moderate heterogeneity and no evidence for publication bias for the outcomes.
Compared with placebo, ACEIs were associated with significant increased risk of angioedema (RR 2.29, 95% CI 1.02 to 5.15), hypotension (RR 1.40, 95% CI 1.22 to 1.62), and renal insufficiency (RR 1.29, 95% CI 1.02 to 1.63; Supplementary Figures 3–5 available online only). Compared with active controls, ACEIs were associated with significant increased risk of angioedema (RR 2.79, 95% CI 1.05 to 7.42, p = 0.04), whereas no increased risk of hypotension (RR 0.80, 95% CI 0.57 to 1.12, p = 0.19) and renal insufficiency (RR 0.87, 95% CI 0.70 to 1.07, p = 0.18) was observed ( Table 3 ). The heterogeneity was low, and there was no evidence of publication bias for any outcomes.
| Outcomes | ACEIs vs Placebo | ACEIs vs Active controls | ||||
|---|---|---|---|---|---|---|
| Studies ∗ | Patients | RR (95% CI) | Studies ∗ | Patients | RR (95% CI) | |
| Efficacy | ||||||
| All-cause mortality | 7 | 19,904 | 0.86 (0.77 -0.96) | 10 | 84,848 | 0.99 (0.95 -1.03) |
| Cardiovascular mortality | 7 | 19,904 | 0.81 (0.72 -0.90) | 10 | 84,848 | 0.99 (0.93 -1.04) |
| Myocardial Infarction | 4 | 17,944 | 0.82 (0.75 -0.91) | 9 | 83,991 | 0.94 (0.88 -1.00) |
| Heart Failure | 6 | 19,047 | 0.76 (0.70 -0.83) | 9 | 83,991 | 0.97 (0.91 -1.03) |
| Stroke | 5 | 17,305 | 0.88 (0.65-1.19) | 9 | 84,126 | 1.07 (0.99-1.15) |
| Safety | ||||||
| Angioedema | 3 | 15,059 | 2.29 (1.02 -5.15) | 5 | 66,492 | 2.79 (1.05 -7.42) |
| Hypotension | 5 | 14,135 | 1.40 (1.22 -1.62) | 5 | 34,785 | 0.80 (0.57 -1.12) |
| Renal insufficiency | 4 | 4,838 | 1.29 (1.02 -1.63) | 5 | 62,665 | 0.87 (0.70 -1.07) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


