Atrial fibrillation (AF) is common in patients on dialysis. Although randomized trials of anticoagulation for AF have demonstrated striking reductions in stroke, these trials did not recruit patients on dialysis. We thus undertook this systematic review and meta-analysis of observational studies including patients with AF on dialysis that reported associations of anticoagulation use. Twenty studies involving 529,741 subjects and 31,321 patients with AF on dialysis were identified. Anticoagulation was associated with a 45% (95% CI 13% to 88%) increased risk of any stroke, reflecting a nonsignificant 13% (95% CI −4% to 34%) increased ischemic stroke risk and 38% (95% CI 3% to 85%) increased hemorrhagic stroke risk. There was also a 44% (95% CI 38% to 56%) lower risk of any thromboembolism, and a 31% (95% CI 12% to 53%) increased risk of any bleeding but no clear association with cardiovascular death (relative risk 0.99, 95% CI 0.86 to 1.15) or all-cause mortality (relative risk 0.97, 95% CI 0.90 to 1.04). Incident event rates were similar or worse in patients on anticoagulation. In conclusion, these observational analyses provide little supporting evidence of benefit, and instead suggest harm, from anticoagulation in patients on dialysis with AF. These results raise the possibility that the effects of anticoagulation in patients with AF on dialysis may not be similar to the clear benefit of anticoagulation seen in patients with AF without end-stage renal disease. Randomized trials are required to definitively evaluate the safety and efficacy of anticoagulation for AF in the dialysis setting.
Atrial fibrillation (AF) is increasingly common in patients with end-stage renal disease on dialysis, which is concerning as AF confers significant additional morbidity and mortality. Randomized trials in general populations with AF have demonstrated that anticoagulation decreases ischemic stroke to a larger extent than any increase in hemorrhagic stroke, resulting in a two-thirds reduction in any stroke. Given these large effects of treatment, the significant benefit of anticoagulation can also be demonstrated in observational data from general populations with AF, despite the potential for moderate biases in such nonrandomized data. However, the aforementioned randomized trials largely excluded patients with end-stage renal disease on dialysis, and some observational studies have suggested excess bleeding and even ischemic hazards from anticoagulation for AF in the dialysis setting. In an attempt to provide further insight in this area, we undertook this meta-analysis to assess whether the available observational evidence supports a benefit from anticoagulation for patients with AF on dialysis, commensurate with that seen from both randomized and observational evidence in general populations with AF.
Methods
Full details of the methods are presented in the Appendix . In brief, we performed a systematic search of observational studies in MEDLINE and EMBASE databases up to June 2015. Eligible studies included patients with AF on dialysis reporting outcome data according to vitamin K antagonist use (either measures of relative risk [RR] for the association between anticoagulation use and outcomes) or incident event rates according to anticoagulation use. We sought to include patients on either hemodialysis or peritoneal dialysis although we permitted studies reporting data on combined renal replacement therapy cohorts that included a minimal proportion of kidney transplants (<10%). Outcomes of interest included any thromboembolism (ischemic stroke or peripheral embolism), any stroke, ischemic stroke, hemorrhagic stroke, major bleeding, cardiovascular death, and all-cause mortality. Studies with prospective cohort, retrospective cohort, or nested case-control study designs were eligible for inclusion.
Data from included studies were extracted in duplicate using a standard protocol. Data were extracted on study characteristics (study period, country, cohort source, design, follow-up, and risk of bias), participant characteristics (number, age, and gender), treatment use (dialysis type, anticoagulation use, and antiplatelet use), and covariates adjusted for in multivariate models.
Risk estimates and CIs on the associations between anticoagulation and study outcomes were extracted from each study where available and log-transformed. We preferentially extracted maximally adjusted RRs from each study. Event numbers, person-time and incident rates per 100 person-years were also abstracted or calculated where available. Exact methods based on the Poisson distribution were used to calculate CIs for rates if necessary. Summary estimates for associations and event rates were subsequently calculated using meta-analytic techniques. The main meta-analytic approach used in our analyses was to take the simple inverse-variance–weighted average of the like-with-like comparisons within each study; sensitivity analyses were also undertaken using a random-effects approach. Between-study heterogeneity was assessed using I 2 statistics. Factors potentially contributing to heterogeneity were explored through sensitivity analyses, subgroup analyses, and meta-regression techniques; these included study characteristics (study period, country, cohort source, design, follow-up, and quality/risk of bias), participant characteristics (number, age, and gender), treatment use (dialysis type, anticoagulation use, and antiplatelet use), and covariate adjustment. We screened for small-study effects that might be attributable to publication bias using funnel plots of effect size plotted against standard error, Egger’s test, Begg’s test; where present, the effect of these was characterized using trim and fill procedures. Only, overall RRs have 95% CI; all other RRs have 99% CIs. A 2-tailed value of p <0.05 was considered statistically significant, and all analyses were performed using Stata, version 13.0 (Stata Corporation, College Station, Texas) and R version 3.2.1 (R Project for Statistical Computing, Vienna, Austria).
Results
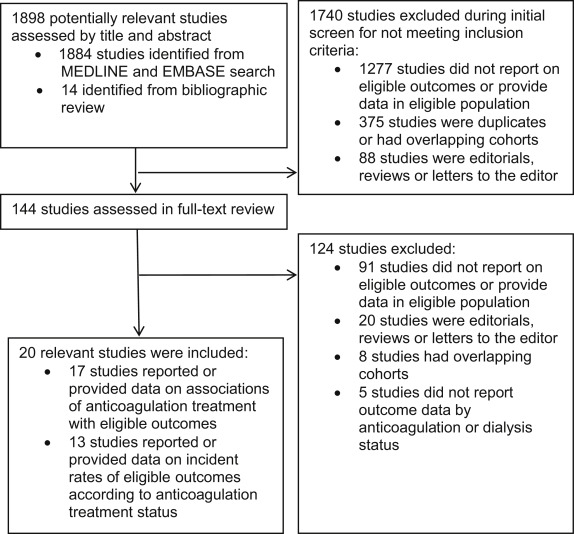
The systematic search of electronic databases identified 1,884 studies, and an additional 6 studies were identified by manual searching of reference lists ( Figure 1 ). From these, we identified 144 potentially relevant studies for full-text review. A total of 20 studies were subsequently included, involving 529,741 subjects and 31,321 patients with AF on dialysis ( Table 1 and Supplementary Tables 1 and 2 ). Of the these studies, 17 reported or provided data on associations of anticoagulation treatment with study outcomes, and 13 reported or provided data on absolute incident event rates according to anticoagulation treatment status. Three were prospective cohort studies, and 17 were retrospective cohort studies. The vast majority of patients were on hemodialysis; 4 studies included a minority of patients on peritoneal dialysis, and 2 studies included a minimal proportion of kidney transplants.
| Reference | Study period | Country | Cohort source | No. of patients | Dialysis type, % | Follow up, y | Mean age, y | Men, % | VKA use, % | AP use, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbott (2003) | 1996-2000 | United States | USRDS DMMS Wave II | 123 | 100 HD | 2.92 | 58.9 | 53.4 | 5.9 | 18.6 |
| Bonde (2014) | 1997-2011 | Denmark | Nationwide analysis | 1,728 | 72.0 HD, 25.2 PD | 1.65 | 66.8 | 35.0 | 22.8 | 32.7 |
| Chan (2009) | 2003-2004 | United States | FMCA ESRD database | 1,671 | 100 HD | 1.60 | 72.3 | 57.0 | 44.7 | 48.7 |
| Chao (2014) | 1996-2011 | Taiwan | NHIRD database | 10,999 | 100 HD | 1.08 | 71.0 | 46.2 | 0 | 0 |
| Chen C (2014) | 2001-2007 | Taiwan | NHIRD database | 3,695 | 100 HD | 3.81 | NA | NA | 15.4 | NA |
| Chen J (2014) | 1997-2008 | Taiwan | NHIRD database | 4,899 | 100 HD | 4.12 | NA | 46.5 | 6.0 | 33.1 |
| Das (2011) | 2005-2009 | United Kingdom | Royal Berkshire Hospital, Reading | 48 | 100 HD | 4.00 | 70.9 | NA | 41.7 | 54.2 |
| Garg (2015) | 2009-2012 | United States | Beaumont Health System, Michigan | 302 | 100 HD | 3.00 | NA | NA | 39.4 | NA |
| Genovesi (2015) | 2010-2012 | Italy | Multiple dialysis centers | 290 | 100 HD | 2.00 | NA | 60.0 | 46.2 | 47.9 |
| Khalid (2013) | 2005-2010 | United States | Henry Ford Health Systems | 96 | 100 HD | 1.00 | 77.2 | 68.8 | 35.4 | 25.0 |
| Knoll (2012) | 2000-2007 | Austria | INVOR Study | 52 | 86.5 HD, 13.5 PD | 2.84 | 69.1 | 67.3 | 57.7 | 53.8 |
| Lai (2009) | NA | United States | Winchester Medical Centre, New York | 93 | 100 HD | 2.30 | 74.7 | 67.9 | 54.8 | 41.4 |
| Olesen (2012) | 1997-2008 | Denmark | Nationwide analysis | 901 | 78.0 HD, 15.4 PD | 12.00 | 66.8 | 66.4 | 24.8 | 22.00 |
| Phelan (2011) | 2000-2008 | Ireland | Beaumont Hospital, Dublin | 70 | 100 HD | 2.02 | 69.9 | 52.9 | 100 | NA |
| Shah (2014) | 1998-2007 | Canada | Multiple hospitals, Quebec &Ontario | 1,626 | NA | 9.00 | 75.2 | 61.0 | 46.5 | 28.1 |
| Vazquez (2000) | NA | Spain | Hospital General de Especialidades, Ciudad de Jaen | 26 | 100 HD | 1.00 | 59.9 | 54.7 | 7.7 | NA |
| Wakasugi (2014) | 2008-2011 | Japan | Multiple centers in Japan | 60 | 100 HD | 1.83 | 68.1 | 65.0 | 46.7 | 53.3 |
| Wang (2014) | 2000-2008 | New Zealand | Middelmore Hospital, Auckland | 141 | NA | 3.40 | NA | NA | 41.8 | NA |
| Winkelmayer (2011) | 1994-2006 | United States | Medicare beneficiaries database | 2,313 | 95.6 HD, 4.4 PD | 1.93 | 69.9 | 42.5 | 10.8 | NA |
| Wizemann (2010) | 1996-2004 | International | DOPPS Study | 2,188 | 100 HD | 8.00 | NA | NA | 16.0 | 31.0 |
Thirteen studies providing data on incident event rates according to anticoagulation treatment included 24,335 dialysis patients with AF followed up for 72,777 person-years ( Table 2 ). Substantial event rates were noted for most outcomes; for example, we observed total stroke rates of 7.65 per 100 person-years, major bleeding rates of 11.35 per 100 person-years, and all-cause mortality rates of 17.27 per 100 person-years in those on anticoagulation. Of note, incident event rates were generally similar or greater in patients treated with anticoagulation. Few studies provided event data for the outcomes of any thromboembolism or cardiovascular death.
| Outcome | No. of studies | No. of person-years | Incidence rate (per 100 person-years) | 95% Confidence Interval | I 2 Heterogeneity | P Value for Heterogeneity |
|---|---|---|---|---|---|---|
| Any thromboembolism | ||||||
| With anticoagulation | 2 | 270 | 3.70 | 2.45-4.95 | 0.0% | 0.94 |
| Without anticoagulation | 2 | 336 | 3.77 | 2.53-5.02 | 83.3% | 0.014 |
| Any stroke | ||||||
| With anticoagulation | 3 | 1,761 | 7.65 | 6.52-8.78 | 92.3% | <0.001 |
| Without anticoagulation | 3 | 20,165 | 4.01 | 3.24-4.78 | 92.4% | <0.001 |
| Ischemic stroke | ||||||
| With anticoagulation | 8 | 10,081 | 3.11 | 2.82-3.40 | 92.4% | <0.001 |
| Without anticoagulation | 8 | 44,438 | 2.15 | 1.97-2.33 | 94.2% | <0.001 |
| Hemorrhagic stroke | ||||||
| With anticoagulation | 3 | 1,944 | 1.46 | 0.93-1.98 | 77.9% | 0.011 |
| Without anticoagulation | 4 | 17,690 | 0.83 | 0.46-1.20 | 23.4% | 0.27 |
| Major bleeding | ||||||
| With anticoagulation | 5 | 7,684 | 11.35 | 10.74-11.95 | 87.9% | <0.001 |
| Without anticoagulation | 6 | 24,212 | 7.62 | 7.14-8.10 | 97.2% | <0.001 |
| Cardiovascular death | ||||||
| With anticoagulation | 1 | 80 | 1.25 | 0.03-6.96 | NA | NA |
| Without anticoagulation | 1 | 112 | 0.96 | 0.02-5.36 | NA | NA |
| All-cause mortality | ||||||
| With anticoagulation | 4 | 880 | 17.28 | 15.06-19.49 | 98.5% | <0.001 |
| Without anticoagulation | 4 | 4,466 | 13.65 | 11.66-15.63 | 98.6% | <0.001 |
Significant between-study heterogeneity was present for some of these pooled estimates ( Table 2 ), which we attempted to explore through subgroup, sensitivity and meta-regression analyses. Subgroup analyses suggested that ischemic stroke and bleeding rates may vary by region ( Supplementary Table 3 ), but no other consistent differences were observed in subgroup analyses, nor did sensitivity analyses or meta-regression suggest any other factors contributed to heterogeneity. Moderately higher incident event rates for ischemic stroke were also noted in sensitivity analyses using random-effects meta-analysis ( Supplementary Table 4 ), but otherwise no material differences were noted. Although there was some evidence of possible publication bias for the outcome of ischemic stroke rates ( Supplementary Figure 1 ), trim and fill procedures accounting for these had no material effect on the summary estimate.
Seventeen studies providing data on associations of anticoagulation treatment with study outcomes included 20,490 patients with AF on dialysis followed up for 90,746 person-years. Anticoagulation treatment was associated with a 45% (RR 1.45, 95% CI 1.13 to 1.88) greater risk of any stroke and nonsignificant 13% (RR 1.13, 95% CI 0.96 to 1.34) greater risk of ischemic stroke, and 38% (RR 1.38, 95% CI 1.03 to 1.85) greater risk of hemorrhagic stroke ( Figures 2 and 3 ). There was a 40% (RR 0.60, 95% CI 0.45 to 0.79; Figure 3 ) lower risk of any thromboembolism and 31% (RR 1.31, 95% CI 1.12 to 1.53; Figure 4 ) increased risk of any bleeding. No clear association of anticoagulation treatment was observed with cardiovascular death (RR 0.99, 95% CI 0.86 to 1.15) or all-cause mortality (RR 0.97, 95% CI 0.90 to 1.04; Figure 5 ).
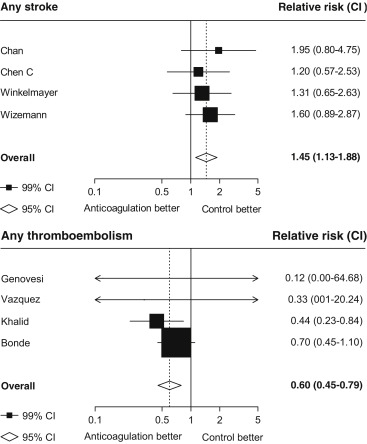
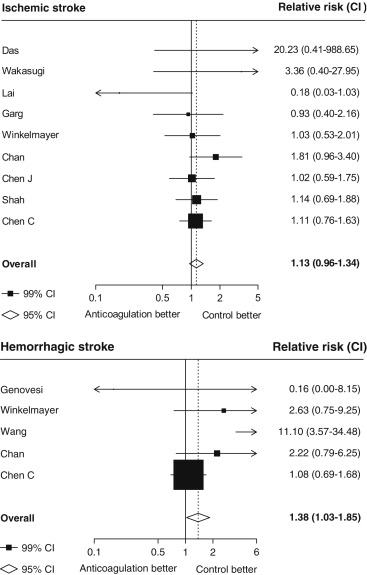
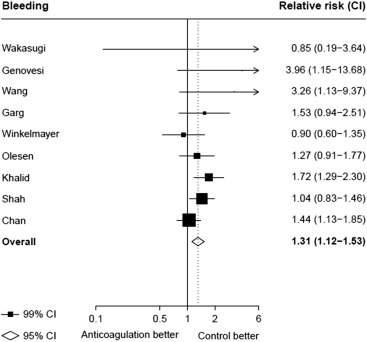
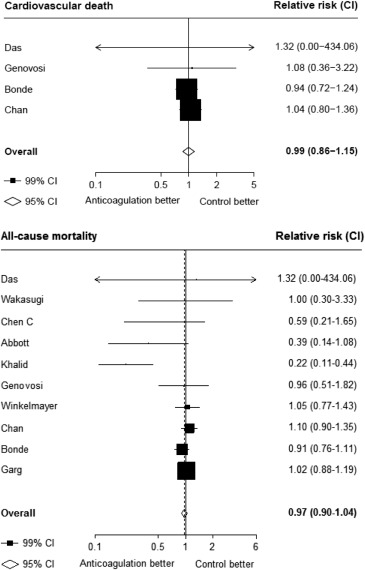
Between-study heterogeneity was observed for summary estimates of the associations of anticoagulation treatment with ischemic stroke (I 2 statistic = 55.6%, p = 0.021), hemorrhagic stroke (I 2 statistic = 71.1%, p = 0.008), (I 2 statistic = 76.3%, p <0.001) but not for the other outcomes of any stroke, any thromboembolism, and bleeding and cardiovascular death (p >0.05 for all). Although excluding some individual studies in sensitivity analyses reduced some of this heterogeneity, no material change in overall summary estimates resulted ( Supplementary Table 5 ). In sensitivity analyses using random-effects meta-analysis, moderately stronger summary estimates resulted for the associations of anticoagulation treatment with hemorrhagic stroke and all-cause mortality; otherwise, no material differences were seen ( Table 3 ). Other sensitivity, subgroup and meta-regression analyses did not suggest other identifiable study or population characteristics significantly contributed to heterogeneity. There was no evidence of publication bias for any of these associations (p >0.1 for all).



