Chapter 32 Mechanistic and Methodological Considerations for the Imaging of Mental Stress Ischemia
INTRODUCTION
The role of emotion or stress in the provocation of angina pectoris has been described as far back in ancient history as Celsus, followed by Hunter in the 1700s, and by Osler at the turn of the 20th century.1 We have an intuitive sense of the importance of these factors, yet gathering empirical data to support or refute this intuition have been slow to unfold. The effort to do so has been hindered by the impact of the philosophical proposition of the duality of the mind and body entrenched in Western culture since Descartes, and by the compartmentalized structure of biomedical research.2 Modern epistemological/ontological constructs and emerging studies in neuroscience have questioned this duality. The latter enjoined with the expansion of integrative biomedical research will shed further light on the interaction of emotional processing/triggers and biological consequences in the next decade.
In the few short years since the previous edition of this volume, new information regarding the nature of mental stress–provoked ischemia, with refinement of key aspects of the pathophysiologic construct, has emerged. In this edition, we discuss new advances in diagnostic tools available to improve the accuracy of mental stress ischemia (MSI) diagnosis, such as peripheral arterial tonometry (PAT). We review MSI as a vulnerability factor for lethal arrhythmias and arrhythmia-induced implantable cardioverter defibrillator (ICD) shocks. We also describe the phenomenon of myocardial stunning due to sudden emotional stress (variably known as takotsubo cardiomyopathy or “broken heart syndrome”) as part of the continuum described by stress-provoked cardiac phenomena. In the previous edition, we outlined the importance of parasympathetic withdrawal and endothelial dysfunction in response to stress or emotionally provoked ischemia. We will discuss the possible links between these two consistent observations of MSI (parasympathetic withdrawal and endothelial dysfunction), based upon studies describing a new pathway that may mediate the vascular effects of MSI, termed the cholinergic antiinflammatory reflex.3
MENTAL STRESS ISCHEMIA
Myocardial ischemia that occurs in response to mental and emotional stress—MSI—is a phenomenon of clinical importance associated with a threefold increased risk of poor outcome in patients with coronary artery disease (CAD).4–9 The pathophysiology of MSI demonstrates a degree of overlap with that of demand-induced ischemia; however, MSI has distinct physiologic correlates, as suggested by differences in hemodynamic, vascular, and neuroendocrine responses to these distinct forms of stress.10,11 In addition, certain psychological factors related to the experience and expression of anger and hostility identify patients who are more vulnerable to MSI.12–15 The history of nuclear cardiology is replete with examples of our creativity in addressing difficult diagnostic challenges in order to demonstrate the manifestation of occult myocardial ischemia. Myocardial ischemia provoked by mental and emotional stress is pervasive and an important presentation within the spectrum of CAD. The overarching goal of this chapter is to provide a working conceptual construct grounded in empirical data on which to base future noninvasive testing for the identification of those at risk for MSI.
ECG Changes During Mental Stress
The ability to detect myocardial ischemia under real-life situations was made possible by the development of the Holter monitor in the 1960s, which records the ECG in real time on a medium that provides for later examination of a depression in the ST-segment—the marker of ischemia. By the latter part of that decade, cardiologists were using this technology to monitor coronary patients while they drove their car, while they engaged in routine daily activities, and while they performed stressful tasks in the laboratory. Studies using this approach demonstrated that among patients with CAD, ischemia is common during moderate to extreme mental and emotional activity independent of the degree of concurrent physical exertion,15–18 is most usually without symptoms of angina, and accounts for up to 75% of total ischemic burden (Fig. 32-1).14,16,19 Most ischemic episodes during mental stress were found to occur at lower heart rate and blood pressure than episodes during physical exertion, supporting the concept of dynamic coronary obstruction (i.e., ischemia caused by changes in arterial tone) as a pathophysiologic mechanism. This does not imply, however, that myocardial demand is not a contributor to MSI as well, since increases in diastolic blood pressure in particular are often seen.20
Left Ventricular Dysfunction During Mental Stress
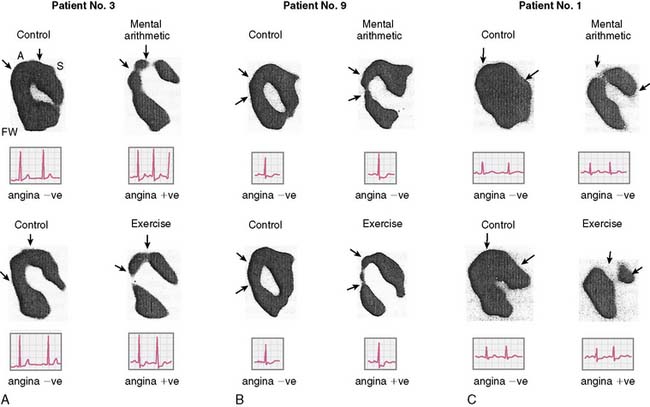
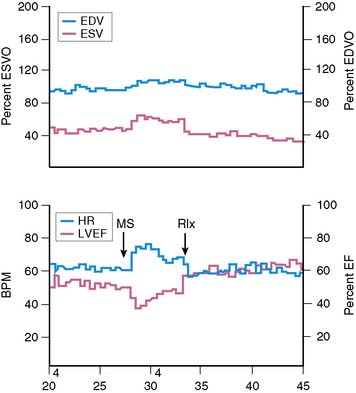
Assessment of left ventricular ejection fraction (LVEF) provides a sensitive (but potentially less specific) method to evaluate the acute cardiovascular effects of various interventions under controlled conditions. The development of radionuclide- and ultrasound-based methods to assess LVEF provided the opportunity to more directly examine the effects of stress on myocardial function as an index of ischemia, and marked the next step in the emergence of this research. The approaches used for this purpose have included equilibrium radionuclide angiography (ERNA), echocardiography, and volumetric measurement with a nonimaging nuclear probe. ERNA has been used in conjunction with mental stress for determination of LVEF and wall-motion abnormalities (WMAS). This approach is similar to that used for exercise ERNA studies, which are no longer routinely performed. In addition to this more standard approach, left ventricular (LV) function during mental stress has been assessed with a nonimaging nuclear probe after injection of radiolabeled red blood cells. This method has the advantage of providing beat-to-beat global LV volume curves, allowing assessment of rapid changes in LV function over an extended period (Fig. 32-2). The disadvantages are that regional wall motion cannot be assessed, and the technology is not widely available. The criteria used to define MSI by these techniques are similar to those used for other forms of stress: a reduction in LVEF of 5% or more and/or the development of a new regional WMA.
Overall, studies conducted in laboratory settings using such methods clearly established that mental stress—induced by having the patient perform any one of several mentally/emotionally demanding tasks—induced transient global and/or regional LV dysfunction in upwards of 50% of patients with CAD. Among patients who demonstrated this dysfunction, it occurred rapidly after the initiation of the stressful task, usually within 1 minute, and recovery was temporally related to the discontinuation of the task and likewise tended to occur rapidly.21 Consistent with the ambulatory ECG data, these abnormalities developed at a lower heart rate than with exercise, were usually asymptomatic, and occurred predominantly, although not exclusively, in patients with exercise tests that were positive for ischemia.12
Studies Using Radionuclide Angiography
Rozanski and colleagues12 were among the first to describe mental stress–induced LV dysfunction by ERNA in a study of CAD patients and normal controls during mental stress and exercise. Largely asymptomatic WMAs were observed during mental stress in 59% of the CAD patients, and 36% had a decrease in ejection fraction of more than 5%. This occurred at a lower heart rate than ischemia induced during exercise, and ECG changes indicative of ischemia were rarely seen. These researchers using the same criteria subsequently found MSI in up to 75% of CAD patients with exercise-induced ischemia. In other studies using only changes in global LV function as evidence of MSI (decrease in LVEF), 50% to 60% of CAD patients demonstrated global LV dysfunction during mental stress.22,23 These changes were usually without ECG changes and—again—asymptomatic.
Studies Using Echocardiography
Data from studies using echocardiography to assess changes in LV function during mental stress have been largely consistent with data from radionuclide-based studies. For example, Modena and colleagues24 reported mental stress–induced LV dysfunction in 38% of patients with chest pain who were referred for angiography. Gottdiener and colleagues25 similarly reported over 50% of patients with known CAD had new regional WMA during mental stress echocardiography. Patients with MSI defined in this manner also showed more frequent ischemia by ambulatory ECG monitoring during sedentary activities.
Despite evidence of LV dysfunction induced during mental stress, a number of unresolved issues remained. For example, the specificity of the LV response as an index of ischemia during mental versus physical stress was unclear. Mental stress causes a substantial sympathetic response that leads to an increase in systemic vascular resistance,11 which may affect LV function independent of ischemia. Indeed the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study10 found that 41% of older normal subjects (43 to 73 years old) exhibited a decrement in LVEF of 5% during mental stress. The reproducibility of LV functional changes during mental stress was and remains a second unresolved issue. The PIMI study data suggest that approximately two-thirds of patients have consistent responses when studied on different days, with the observed reproducibility a function of the type of stressor utilized.26 Others have corroborated these findings.27 A third issue concerned the association between LV functional changes and other potentially more specific indicators of ischemia, such as myocardial perfusion defects. These issues have implications for the development of standardized approaches for risk stratification in the clinical setting.
Myocardial Perfusion During Mental Stress
In an early clinical application of positron emission tomography (PET), Deanfield and colleagues14 found that 75% of patients with positive exercise tests had regional perfusion abnormalities during mental stress. Although the rate-pressure product was lower with mental stress than with exercise, the degree and location of perfusion defect were largely similar between the two forms of stress. A subsequent study by others using planar technetium-99m 99mTc sestamibi perfusion imaging found that 85% of CAD patients had myocardial perfusion abnormalities in the same vascular territory during both mental and physical stress, though the extent and degree of abnormalities were smaller during mental stress.28 These studies, using qualitative methods for assessment of myocardial perfusion, demonstrated that regional heterogeneity of blood flow develops in many patients during mental stress.
Quantitative assessment of myocardial blood flow by PET provides particular insights regarding the differential pathophysiology of MSI. This approach allows investigation of effects that may be more subtle than can be observed by more conventional approaches to assessment of LV function or myocardial perfusion. Several groups of investigators have used this approach to assess the effect of mental stress on absolute myocardial blood. For example, studying CAD patients and normal subjects, Schoder and colleagues found that despite similar increases in rate-pressure product in both cohorts, the magnitude of flow increase during mental stress was less in the patients.29 Subsequently, our group investigated regional differences in blood flow response to mental versus pharmacologic stress in CAD patients and found that with mental stress, a blunted flow response was observed, primarily in regions without significant epicardial coronary stenosis.30 These findings suggest that mental stress may more predominantly affect coronary microvascular tone, implicating this as an operative factor in the pathogenesis of myocardial ischemia at relatively low levels of work.
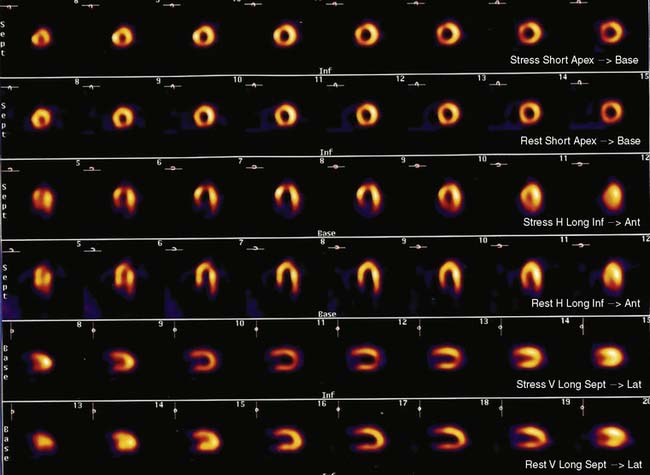
Flow heterogeneity during mental stress may be mild, variable, and perhaps insufficient to generate regional wall motion. Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI), less costly than approaches that rely upon PET, may therefore represent a best and most cost-effective approach to the diagnosis of MSI. Indeed, there is a growing body of research using this modality to study the effects of mental stress. It remains to be determined, however, whether this approach will result in greater sensitivity/specificity in the detection of MSI or incremental prognostic value for patients with CAD. In addition, the relationship of perfusion defects to LV dysfunction during mental stress is unclear. For example, our group has simultaneously assessed perfusion (using SPECT sestamibi) and function (using echocardiography), and has found a significant discordance between perfusion and functional changes during mental stress.31 Further study is needed to assess the significance of these findings. Nonetheless, MPI defects are a reliable indicator of ischemia (Fig. 32-3).
Comparison Between Mental Stress Imaging and Standard Exercise and Pharmacologic MPI
Although both mental stress and exercise induce hemodynamic changes, differences exist in the magnitude of change between these stressors. For example, the increase in heart rate during mental stress is much less than that during exercise.11,12 Systolic blood pressure response can be less than or similar to that during exercise, whereas diastolic blood pressure increases more during mental stress.11,12 Additionally, the rate of increase in the rate-pressure product is accelerated with mental stress and can peak within 1 minute of the onset of stress.21 These differences in hemodynamic responses likely relate to differential neurohormonal activation and peripheral vascular effects. For example, during mentally stressful tasks, epinephrine levels increase more than norepinephrine levels. The opposite is observed during exercise.32 Peripheral vascular resistance decreases during exercise but increases or remains constant during mental stress.11,33 The observation that MSI occurs at relatively low rate-pressure product suggests that other mechanisms such as coronary vasoconstriction and the neurohormonal effects on endothelium-dependent vasomotor tone may be operative. The importance of these findings to any differential pathophysiology between mental stress and exercise-induced ischemia, however, remains to be determined.
Recent studies suggest that vulnerability to either exercise or mental stress–provoked ischemia in the same individual does not reliably predict ischemia to both stimuli. For example, Ramachandruni et al.34 found that 6 of 21 CAD patients (29%) who had previously shown no flow defect during exercise or pharmacologic provocation had a reversible flow defect with mental stress. Conversely, as described earlier, only 40% to 60% of CAD patients with exercise-induced or pharmacologically induced ischemia have MSI. More recently, Hassan et al.35 demonstrated significant intraindividual variability in the severity and location of myocardial ischemia provoked by mental versus exercise or pharmacologic stress. This study of 187 patients with CAD found only 71% concordance for the provocation of ischemia between mental versus exercise/pharmacologic stress. In addition, 11% of the cohort demonstrated ischemia to mental but not exercise/pharmacologic stress, while 22% demonstrated ischemia to exercise/pharmacologic but not mental stress. Furthermore, the location of flow defect(s) provoked by mental versus exercise/pharmacologic stress was often different, as was the severity of the flow defect(s) observed. A prior study by Arrighi et al.30 using regional PET MPI may shed some light on the pathophysiology underlying the Hassan group’s results. In their study, Arrighi and colleagues found that pharmacologic stress caused an expected absolute reduction of myocardial blood flow in regions with significant epicardial disease (compared to regions without significant epicardial disease), and that this decreased flow was associated with a compensatory reduction in distal coronary microvascular resistance. Conversely, during mental stress, absolute myocardial blood flow was lower in regions without significant epicardial disease than in regions with significant epicardial disease, and was associated with an increase in distal coronary microvascular resistance. The study by Arrighi et al. therefore provides insights into the Hassan et al. findings by suggesting a prominent role for coronary microvascular dysfunction in mental versus exercise/pharmacologic stress–provoked ischemia. Overall, these studies describe contrasts in the coronary vascular response to mental stress and traditional clinical provocations to myocardial ischemia. Differences in CNS activity for these discordant groups are emerging and will be discussed under the heading “Functional PET Brain Imaging.”
Prognostic Significance of Myocardial Stress Ischemia
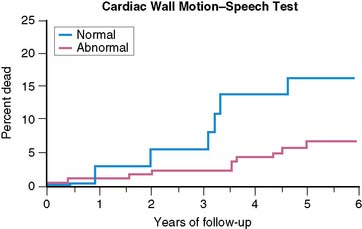
The prognostic significance of MSI has been explored by a number of investigators. In the first published report,33 there were a significantly greater (P < 0.025) number of events (MI, unstable angina) at 2 years in a group of patients who had previously demonstrated LV dysfunction—assessed by radionuclide methods—during mental stress than in the group who did not show this response. In a later study,36 patients with LV dysfunction—by ERNA—during mental stress were at increased 2-year risk of events (MI, unstable angina, revascularization; risk ratio = 2.40), even after adjusting for age, history of MI, and baseline EF. Similarly, in patients followed over a 4.4-year follow-up period (median = 3.5 years),37 almost 45% of patients with MSI in the lab—defined by WMA on ECHO or ERNA—experienced an event (death, MI, unstable angina), while less than 25% of patients without MSI experienced an event. Most recently, the PIMI investigators have reported on the prognostic significance of MSI in their multicenter study,38 with 17 patients dying during an average follow-up of 5.2 years (Fig. 32-4). MSI—defined by WMA on ERNA—had been demonstrated among 40% of those who died but only 17% of those who survived (rate ratio = 3.0; P < 0.04). Other indicators of ischemia during MS testing, including LVEF and/or ECG changes, did not predict death.
Psychological Factors and Prognosis in Mental Stress Ischemia
The contribution of psychological makeup to prognosis in patients with CAD deserves special mention inasmuch as factors including depression, anxiety, anger, and hostility have broadly been reported. Determination of the specific psychological factors that contribute to risk for MSI may help to determine when mental stress testing is likely to provide important prognostic information. Studies have found, for example, that patients with easily provoked anger and hostility have twice the incidence of CAD and a fivefold increased risk of recurrent MI over an extended follow-up period.37 High levels of hostility have also been associated with a higher incidence of restenosis after angioplasty38 and rapid progression of CAD.39 Similarly, chronic anger is associated with a 2.7 relative risk of cardiovascular death, myocardial infarction, and angina,40 whereas the experience of moderate to extreme anger increases risk of MI 2.5-fold for up to 2 hours.41
Data from our group and others specifically related mental stress–induced LV dysfunction to psychological makeup. In comparing patients with MSI to those without MSI, we found no difference on standard clinical indicators, on measures of cardiovascular performance during mental stress testing and exercise thallium testing, or on measures of CAD severity. Patients with MSI, however, scored higher on measures of emotional arousability, hostility, anger, and aggressive response to perceived provocation, and lower on a measure of anger control. In addition, the duration of ischemia evidenced during a clinical interview used as a measure of emotional arousability was highly correlated with the degree of emotional arousability measured by the interview (0.70, P < 0.0001). Hence, a hostile, angry psychological profile was associated with emotional arousability, which increased the risk of MSI.42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree