Management of Neuromuscular Respiratory Muscle Dysfunction
INTRODUCTION
Patients with ventilatory impairment due to ventilatory muscle dysfunction are often evaluated and managed according to practices developed for patients with chronic lung diseases. However, pulmonary function laboratories, designed primarily for assessment of lung diseases, do not evaluate lung volume recruitment capacities or cough flows that are important in the assessment of patients with inspiratory and expiratory muscle dysfunction. In the setting of ventilatory muscle dysfunction polysomnograms may be misinterpreted as central or obstructive apneas and hypopneas rather than hypoventilation due to inspiratory muscle dysfunction, and continuous positive airway pressure (CPAP) or nocturnal bilevel positive airway pressure (BiPAP) prescribed. In the context of ventilatory muscle dysfunction CPAP does not increase tidal volumes, and may actually reduce them by causing them to approach maximum lung capacity for these patients with severe pulmonary restriction while BiPAP is often used at pressures inadequate to support alveolar ventilation, provide inspiratory muscle rest, or assist in coughing.1 In addition, the patients are often treated with supplemental oxygen to correct hypoxemia when efforts to improve oxygenation should be directed at normalizing alveolar ventilation and clearing airway secretions. With advancing inspiratory and expiratory muscle weakness, the common scenario is that respiratory failure ensues and is treated by mechanical ventilation via endotracheal intubation. When ventilator weaning fails, a tracheostomy is performed and mechanical ventilation is continued indefinitely, often in an institution.
Therapeutic modalities commonly used for respiratory diseases can have adverse effects in patients with neuromuscular disorders. Bronchodilator therapy can augment the anxiety and tachycardia that are common in myopathic patients, many of whom have cardiomyopathies. Oxygen therapy increases the risk of pulmonary morbidity, rate of hospitalizations, and mortality by comparison with the use of ventilator assistance or with no treatment at all.2 As noted earlier, oxygen therapy may obscure recognition of mucus plugging because it alleviates oxyhemoglobin desaturation without attention to the expulsion of airway mucus. Oxygen therapy may also prolong hypopneas and apneas during rapid eye movement (REM) sleep,3 and it appears to suppress the reflex muscular activity needed for effective noninvasive intermittent positive pressure ventilation (IPPV) during sleep.4 Translaryngeal intubation, tracheostomy, and tracheal suctioning continue to be used for patients with neuromuscular diseases even though noninvasive IPPV, noninvasive suctioning, and mechanically assisted coughing (MAC) can be more effective and comfortable.
Despite the proven effectiveness of measures to support ventilation noninvasively for long periods even in the absence of respiratory muscle function, these therapeutic modalities have yet to be adopted by many physicians. In Great Britain, 82% of patients with amyotrophic lateral sclerosis (ALS) die receiving morphine and 64% receive benzodiazepines, while few are provided with respiratory muscle aids to prevent respiratory failure.5 This approach both smoothes and hastens passage to the grave by leading to CO2 narcosis.6,7 Often, without consulting the patient, the physician judges the patient’s quality of life to be unacceptable and the disease terminal, ignores options that prevent respiratory complications, renders the patient and family hopeless, and biases the family against ventilator use which the physician associates with tracheostomy. Proclaimed as “palliation,” the results of this professional point of view are anguish and hopelessness and not infrequently lead patients to seek quack therapies and assisted suicide. Over a recent 5-year period, 12 publications in the New England Journal of Medicine concerned clinical management and assisted suicide for patients with ALS. In none of these reports was prevention of respiratory complications or ventilator assistance by invasive or noninvasive means considered. Failure to consider noninvasive IPPV for full ventilator support continues.8,9
PATHOPHYSIOLOGY
Patients with neuromuscular disorders can develop respiratory failure because of some combination of respiratory muscle dysfunction (Table 85-1), that is, dysfunction of inspiratory, expiratory, and bulbar-innervated muscles. These muscle groups will be considered here and the reader is referred to Chapters 84 and 143 for detailed discussions of the physiological disturbances associated with chest wall and neuromuscular disorders that affect ventilation.
TABLE 85-1 Physical Medicine Respiratory Interventions Benefit Patients with the Following Conditions
Autonomously breathing patients with advanced ventilatory muscle dysfunction develop a rapid, shallow breathing pattern with inability to take deep breaths. If untreated, this can lead to chronic microatelectasis and decreased lung and chest wall compliance.10,11 Acute respiratory tract infections with pulmonary scarring and the kyphosis and scoliosis that are common in these patients can cause further loss of lung compliance.
In the context of neuromuscular disorders, hypercapnia develops insidiously as a consequence of shallow breathing as respiratory control centers reset and increasing central nervous system bicarbonate levels accommodate and permit it to worsen. Hypercapnia, itself, can decrease respiratory muscle strength,4 and decreases the effectiveness of its treatment by the nocturnal use of BiPAP or noninvasive IPPV.4 The risk of pulmonary morbidity and mortality from acute respiratory failure correlates with increasing hypercapnia.2,4
Inspiratory muscle weakness, mechanical dysfunction of the chest wall and lungs associated with thoracic deformities, hypopharyngeal collapse or other upper airway narrowing, extreme obesity, abdominal distension, use of improperly fitting thoracolumbar orthoses as well as narcotic and sedative medications, supplemental oxygen, malnutrition and deconditioning, infection, and fatigue can all either cause or exacerbate alveolar hypoventilation and lead to respiratory failure. Oxygen therapy often results in CO2 narcosis, otherwise hypoventilation is usually first recognized during an intercurrent respiratory infection when bronchial mucus plugging triggers pneumonia and acute respiratory failure due to an ineffective cough and fatigue during acute respiratory infections.2,7 Ventilatory failure can also develop suddenly or over a period of hours or days in patients with acute cervical myelopathies, Guillain–Barré syndrome, myasthenia gravis, acute poliomyelitis, or exacerbations of multiple sclerosis.
Patients with generalized muscle dysfunction usually also have concomitant expiratory and oropharyngeal (bulbar) muscle weakness that decreases cough peak flows (CPF). When CPF do not exceed 2.7 L/s, cough may be completely ineffective.12 CPF are reduced by airway obstruction caused by tracheal stenosis, laryngeal incompetence, postintubation vocal cord adhesions or paralysis, hypopharyngeal collapse due to bulbar-innervated muscle weakness or spasticity, airway granulation tissue, and obstructive pulmonary disease. The CPF are reduced further when patients cannot take or receive a breath greater than 1.5 L.13 Thus, the airway secretions that develop during upper respiratory tract infections and after surgical anesthesia often result in pneumonia and acute respiratory failure. Smoking, the presence of an endotracheal cannula that causes bronchorrhea, or bronchorrhea for any other reason increases the tendency to develop mucus plugging that is all too frequently managed by repeated bronchoscopies, intubation, and tracheotomy without trying MAC. Intubation, however, results in a burden of pathogenic bacteria that, even when not causing ventilator-associated pneumonia, exceeds the commonly accepted threshold for diagnosing it.14
For patients with ventilatory muscle dysfunction, arterial hypoxemia and hypercapnia occur initially during REM sleep and later extend throughout sleep and eventually throughout the awake hours. Cough reflex is also suppressed during sleep, which is when mucus plugs are most likely to cause sudden and severe hypoxemia. Normocapnic arterial hypoxemia is also common during sleep, most likely reflecting ventilation/perfusion mismatches associated with microatelectasis, scoliosis, airway mucus congestion, and pulmonary scarring.
 THE RESPIRATORY MUSCLE GROUPS
THE RESPIRATORY MUSCLE GROUPS
The diaphragm is the principal muscle of inspiration. The abdominal muscles are the principal muscles of expiration or coughing. The bulbar-innervated muscles are the muscles of the upper airway. They include the muscles of the mouth, uvula and palate, tongue, and larynx. While these muscles do not have a direct action on the chest wall, they are essential for keeping the upper airway patent; they affect airway resistance and airflow; and they permit glossopharyngeal breathing (GPB).
Decreased inspiratory muscle function results in decreased vital capacity (VC), atelectasis, increased relative work of breathing, and eventually in hypoventilation. Expiratory, inspiratory, and bulbar-innervated muscle dysfunctions result in an ineffective cough. The latter can also affect speech, swallowing, and safe food and saliva management. Fortunately, the inspiratory and expiratory muscles can be substituted for by physical medicine interventions. Indeed, numerous patients with no muscle function below the neck and no measurable VC for over 50 years do not need tracheostomy tubes or develop hypercapnic respiratory failure.
INSPIRATORY AND EXPIRATORY MUSCLE AIDS
Inspiratory and expiratory muscle aids are devices and techniques that involve the manual or mechanical application of forces to the body or pressure changes to the airway to assist or substitute for inspiratory or expiratory muscle function. Negative pressure applied to the airway during expiration assists the expiratory muscles for coughing, just as positive pressure applied to the airway during inhalation (noninvasive IPPV) assists inspiratory function.
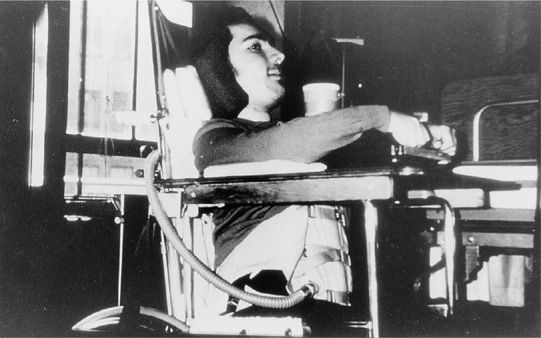
A manual thrust applied to the abdomen during expiration, especially when in combination with mild chest compression, assists expiratory muscle function and increases cough flows.13 The devices that act on the body to enhance inspiratory and expiratory muscle function include body ventilators. The intermittent abdominal pressure ventilator (IAPV) involves the intermittent inflation of an elastic air sac that is contained in a corset or belt worn beneath the patient’s outer clothing (Fig. 85-1). The sac is inflated by a positive pressure ventilator. Bladder action against the abdominal wall moves the diaphragm upward, causing a forced exsufflation. During bladder deflation, the abdominal contents and diaphragm return to the resting position, and inspiration occurs passively. A trunk angle of 70 to 80 degrees from the horizontal is ideal for use. The patient who has any inspiratory capacity or is capable of GPB can add autonomous volumes to the mechanical insufflations. The IAPV generally augments tidal volumes by about 300 mL, but volumes as high as 1200 mL have been reported when there is no scoliosis or obesity.15 Patients with less than 1 hour of ventilator-free breathing ability tend to prefer to use the IAPV rather than use noninvasive IPPV during daytime hours.15
Figure 85-1 The girdle of the intermittent abdominal pressure ventilator (IAPV) with its air sac connected to the tubing of a volume-cycled ventilator. This DMD patient with no measurable vital capacity used the IAPV for daytime ventilator support for 15 years.
Note, CPAP does not assist inspiratory or expiratory muscles and should rarely if ever be used for these patients whose symptoms of sleep disordered breathing are associated with muscle weakness rather than central or obstructive sleep apneas.
CLINICAL GOALS
The goals of management are to optimally inflate the lungs and chest wall to maintain pulmonary compliance, to maintain normal alveolar ventilation around-the-clock, and to maximize CPF.
 MAINTAIN PULMONARY COMPLIANCE AND CHEST WALL MOBILITY
MAINTAIN PULMONARY COMPLIANCE AND CHEST WALL MOBILITY
Incentive spirometry can expand the lungs no greater than the VC so it is ineffective as a tool for lung volume recruitment. Like limb articulations, the lungs and chest wall too require regular mobilization.11 This can be achieved actively by air stacking (Video 85-1),16 passively by providing deep insufflations for those with an incompetent glottis, or by nocturnal noninvasive ventilation for infants who cannot cooperate with air stacking or passive deep insufflations.17
Video 85-1 A ventilator-dependent patient with amyotrophic lateral sclerosis who failed all spontaneous breathing trials, extubated to full-support noninvasive intermittent positive pressure ventilation via a 15-mm angled mouth piece. He demonstrates lung volume recruitment and increased voice volume by air stacking. Access at www.fishmansonline.com
A patient’s maximum insufflation capacity (MIC) is the largest volume of air that can be held with a closed glottis. The patient “air stacks” consecutively delivered volumes from a volume-cycled ventilator or a manual resuscitator, holding these volumes with a closed glottis to maximally expand the lungs. The air is delivered via a mouth piece (Video 85-1), nasal or oronasal interface if the lips are too weak to retain the air. This is performed multiple times in three daily sessions. Patients who learn GPB can often air stack consecutive gulps to or beyond the MIC. The difference between the MIC and the VC is a function of bulbar-innervated muscle integrity (force of glottic closure).13 If the bulbar muscles are too weak for deep active air stacking, single deep insufflations are provided via a mechanical insufflator–exsufflator at 40 to 70 cm H2O in three daily sessions. Deep insufflations can also be delivered via manual resuscitator with the expiratory valve blocked.
The primary objectives of lung recruitment therapy are to increase voice volume and MIC, maximize CPF, improve pulmonary compliance, prevent atelectasis, and master noninvasive IPPV. Occasionally the VC also increases with increases in MIC. Should the situation arise, the ability to air stack facilitates extubation to noninvasive IPPV (Video 85-1). This is extremely important for avoiding tracheotomy because such patients can be safely extubated without being ventilator weaned.18
Lung volume recruitment also promotes lung growth and chest wall development in children. While infants cannot air stack, nocturnal use of high span (IPAP [inspiratory positive airway pressures] – EPAP [expiratory positive airway pressures ] >10 cm H2O) BiPAP or a pressure-cycling portable ventilator set at pressures over 16 cm H2O has been demonstrated to prevent pectus excavatum and promote lung and chest wall growth for infants with spinal muscular atrophy (SMA), all of whom have paradoxical breathing when not using it.19,20
 MAINTAIN NORMAL ALVEOLAR VENTILATION
MAINTAIN NORMAL ALVEOLAR VENTILATION
Noninvasive Ventilation
BiPAP is not optimal for patients with neuromuscular disorders because one cannot air stack using pressure-cycled ventilators or fully expand the lungs with the machines currently on the market. IPPV from volume-cycled machines can be delivered via lip seals, nasal, or oral–nasal interfaces for ventilator support during sleep. The patients can be trained and equipped in the outpatient and home settings. While inpatient polysomnography can be helpful for adjusting ventilator settings to optimize sleep quality, we have managed over 1500 patients on noninvasive ventilation, many of whom eventually requiring it around-the-clock, without using this expensive intervention.
Patients requiring around-the-clock support use simple 15- or 22-mm angled mouth pieces that are grabbed with the teeth for IPPVs (Fig. 85-2) during the day. To use mouthpiece IPPV, adequate neck rotation and oral motor function are necessary to grab the mouth piece and receive IPPV without insufflation leakage out of the mouth or nose. In addition, the patient must open the glottis and vocal cords, dilate the hypopharynx, and maintain airway patency to receive the air.
Figure 85-2 A 48-year old with Duchenne muscular dystrophy, continuously dependent on noninvasive intermittent positive pressure ventilation (IPPV) since 23 years of age, seen here using IPPV via a 15-mm angled mouth piece for daytime ventilator support.
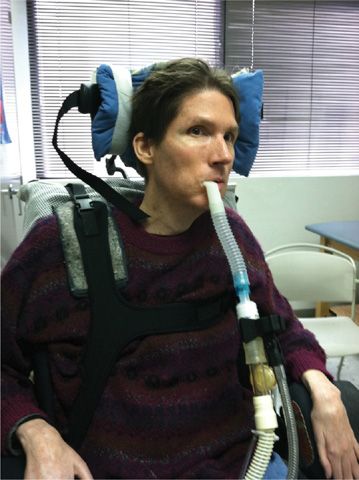
When the lips are too weak to grab a mouthpiece, the patient can use an IAPV15 or continue nocturnal nasal IPPV into daytime hours (Fig. 85-3). In the latter case, nasal interfaces are alternated to vary skin pressure. Inconspicuous nasal interfaces that permit the use of eye glasses can also be used.
Figure 85-3 Brothers with spinal muscular atrophy type 1 (Werdnig–Hoffman disease) who have been continuously noninvasive intermittent positive pressure dependent since 4 months of age using a nasal prongs interface during daytime hours that permits the use of eye glasses. These patients’ lips are too weak to use mouth piece; they have had nasogastric tube feedings since infancy; and their vital capacities have been 0 to 10 mL for more than 10 years but they are managed noninvasively.
Although the use of oronasal interfaces is popular in some centers, we have rarely found them to be necessary. Even patients with little or no measurable VC can be safely ventilated day and night by open systems of nasal or oral ventilation. Closed systems are unnecessary provided that ventilatory drive is not blunted by oxygen therapy, sedative medications, or excessive daytime hypercapnia, all of which can result in excessive air leakage out of the nose or mouth when using the open systems of mouth piece/lip seal or nasal ventilation for sleep.4 If necessary, one can provide an essentially closed system of ventilatory support by using a lip seal device and placing cotton pledgets in the nostrils and sealing the nostrils with a band-aid, or more commonly today, by using a nasal prong system that extends down over the lips, for example, the Hybrid Universal InterfaceTM (Innomed Technologies, Savannah, Georgia) and the Mirage LibertyTM interface (ResMed Inc., Duncan, South Carolina).
The benefits derived from the part-time, usually nocturnal, use of noninvasive ventilation, appear to be due to some combination of respiratory muscle rest, increasing tidal volumes and alveolar ventilation, and improving blood gases, lung compliance, chemotaxic sensitivity, and possibly ventilation/perfusion matching by reducing atelectasis and small airway closure.4 To accomplish optimal rest, high volumes or pressure spans are used, that is, assist-control mode at volumes of 800 to 1500 mL or pressures of 18 to 20 cm H2O for adults and inspiratory-to-expiratory positive airway pressure spans of 13 to 18 cm H2O for BiPAP users. Patients vary the volume of air taken in from ventilator cycle to ventilator cycle to vary tidal volume, speech volume, and cough flows, as well as to air stack and provide lung expansion.
Complications of noninvasive IPPV include claustrophobia, skin discomfort, abdominal distension, occasional allergy to the plastic or silicone interfaces, mouth dryness, eye irritation from air leakage, nasal congestion and dripping, sinusitis and nose bleeds, gum discomfort and receding, maxillary flattening in children, aerophagia,21,22 and, as for invasive ventilation, barotrauma. Switching to lip-delivered IPPV can relieve most of the difficulties associated with nasal IPPV, however, it is more difficult to speak when using lip-delivery devices. Abdominal distention can be alleviated by switching to pressure cycling. Distension is often relieved as the air passes as flatus once the patient sits up in the morning or by “burping” of a gastrostomy tube if present. Barotrauma can occur with invasive or noninvasive ventilation but is rare with the latter for patients with neuromuscular disorders.
 FACILITATE CLEARANCE OF AIRWAY SECRETIONS
FACILITATE CLEARANCE OF AIRWAY SECRETIONS
Chest percussion and vibration can help mobilize deep airway secretions, but they are not substitutes for coughing. Cough can be assisted manually or by mechanical means.
Manually Assisted Coughing
Manually assisted coughing requires substantial lung inflation by air stacking or a deep passive lung insufflation especially if the VC is less than 1.5 L.13 This is followed by an abdominal thrust applied as the glottis opens. Whereas all three respiratory muscle groups are needed for spontaneous coughing, only bulbar-innervated muscle function is required for assisted coughing. This is because airway pressure changes and abdominal thrusts substitute for inspiratory and expiratory muscles but there is nothing noninvasive that can substitute for the function of the glottis.
Manually assisted coughing requires a cooperative patient, good coordination between the patient and care giver, and adequate physical effort and often frequent application by the care giver. When inadequate, and especially when inadequacy is due to difficulty air stacking or diminished strength of the glottis, MAC is required.
Mechanically Assisted Coughing
MAC combines passive mechanical insufflation–exsufflation with an abdominal thrust. Mechanical insufflator–exsufflators deliver deep insufflations followed immediately by deep exsufflations. The MAC cough volumes normally exceed 2 L at flows of 10 L/s. Insufflation to exsufflation pressures of +50 to –50 cm H2O delivered via oronasal interface or adult tracheostomy or translaryngeal tubes with the cuff inflated are most effective. Machine pressures, however, are secondary. What is important is to fully expand and then fully and rapidly empty the lungs.
Whether via the upper airway or via indwelling airway tubes, routine airway suctioning misses the left main stem bronchus about 90% of the time. This explains high rates of left lower lobe pneumonia. MAC, on the other hand, provides the same exsufflation flows in both left and right airways without discomfort, fatigue, or airway trauma and it can be effective when suctioning is not.
MAC supports or takes the place of the inspiratory and expiratory muscles. Thus, the patients who need it are those whose inspiratory and expiratory muscles are too weak for effective coughing but whose bulbar-innervated muscle function can maintain adequate airway patency but not air stack effectively for assisted CPF over 5 L/s. This is typical of most patients with neuromuscular disease. On the other hand, MAC is usually unnecessary for patients with intact bulbar-innervated muscle function such as those with spinal cord injury, as they can usually air stack sufficiently such that with a properly applied abdominal thrust (assisted) CPF can exceed 6 L/s. MAC becomes ineffective if bulbar-innervated muscle function cannot maintain airway patency during the mechanical exsufflation or if saliva is continually aspirated as often happens in advanced bulbar ALS.
THE OXIMETRY FEEDBACK—RESPIRATORY AID PROTOCOL
This protocol consists of using inspiratory (noninvasive IPPV) and/or expiratory (MAC) aids in combination with pulse oximetry feedback to maintain patients’ room air SaO2 ≥95%. The protocol is most important during respiratory tract infections and when extubating patients with little or no VC (Video 85-2). Noninvasive IPPV and MAC with oximetry feedback has averted hundreds of hospitalizations for patients with Duchenne muscular dystrophy (DMD),23 SMA,17 ALS,24 and other neuromuscular conditions.25 On the other hand, tracheostomy is indicated when saliva is continuously aspirated and the SaO2 remains below 95% despite optimal use of noninvasive IPPV and MAC. This essentially only occurs in advanced bulbar ALS. Ninety percent (33 of 35) of these ALS patients whose SaO2 baseline has decreased below 95% despite noninvasive IPPV and MAC were reported to have been deceased within 2 months unless undergoing tracheotomy.15,23,24
Video 85-2 This video demonstrates postextubation airway secretion expulsion by mechanical insufflation–exsufflation (MIE) applied via an oronasal interface. A large bolus of mucopurulent material can be seen entering the interface during the exsufflation phase of MIE. Access at www.fishmansonline.com
NONINVASIVE VERSUS TRACHEOSTOMY IPPV OUTCOMES
In a controlled study in which over a 7-year period all continuously ventilator-dependent DMD patients underwent tracheostomy, 21 survived to a mean age of 28.1 ± 8.3 years of age with 3 still alive. Over the next 21-year period 88 consecutive continuously ventilator-dependent DMD patients used only continuous noninvasive IPPV with none undergoing tracheostomy. These 88 had a 50% survival by Kaplan–Meier analysis to 39.6 years of age (p <0.001).26 We, too, have reported DMD survival by continuous noninvasive IPPV dependence for 101 DMD with 5 now over age 40 and having required continuous support for over 20 years,27 and including one to age 48 having used continuous noninvasive support for 25 years.28 Twenty-two DMD patients unable to pass spontaneous breathing trials were extubated and 8 decannulated to continue full-time noninvasive IPPV.18
Considering ALS, although it appears that all patients would eventually lose sufficient bulbar-innervated muscle function to satisfy the SaO2 criterion for tracheotomy,24 in one study 22 patients (25%)29 and in another 41 (42%)24 became dependent on continuous noninvasive IPPV for means of 8 and 12 months (range to 8 years), respectively, before requiring tracheotomy. In the latter study, 13 became continuously ventilator dependent without developing acute respiratory failure or requiring hospitalization. The difference between the patients who could be spared respiratory failure from those who could not was that the latter had stridor and upper airway spasticity as well as no ability to generate measurable CPF.24 We have extubated 16 and decannulated 5 ALS patients who could not pass spontaneous breathing trials and who subsequently survived as long as 8 years using continuous noninvasive IPPV before requiring tracheotomy. Once bulbar ALS patients undergo tracheostomy for ventilatory support, survival has been reported to be about 5 years before most patients die from complications related to their tracheostomies.30
Infants with SMA type 1 have 70% mortality by 6 months of age and 90% by 24 months of age from respiratory failure unless undergoing tracheotomy. In a recent study of 80 nasal noninvasive ventilation users, all of whom continuously ventilator dependent before 24 months of age, the protocol extubation (Table 85-2) success rate was 87% by comparison to 6% by conventional extubation approaches. Hospitalization rates for the noninvasively managed patients fell from 1.6 per year up to age 3 to 0.04 per year after age 5.17 Fourteen such typical and severe SMA type 1 patients are currently over 15 years of age, with the oldest being 22 years of age, using nasal ventilation 24 hours a day in some cases since 4 months of age (Video 85-3).31 Sixty of 63 typical and severe SMA type 1 patients who required intubation for intercurrent chest infections were successfully extubated to continuous noninvasive support despite being unable to pass spontaneous breathing trials.31 SMA type 1 patients who undergo tracheotomy have also been described to survive into adolescence but tend to die from complications of the tracheostomy tube.17