Management of Left Subclavian Artery with TEVAR
KRISTOFER M. CHARLTON-OUW and ALI AZIZZADEH
Presentation
An 82-year-old woman complains of left-hand pain after undergoing an urgent TEVAR for a symptomatic descending thoracic aortic aneurysm. A preoperative CT scan showed both saccular and fusiform aneurysms of the descending thoracic aorta without evidence of rupture (Fig. 1A and B). The proximal extent of the aneurysm was near the origin of the left subclavian artery (LSA) and had a maximal diameter of 6.0 cm. Isolation of the aortic aneurysms required two overlapping endografts and coverage of the LSA (Fig. 2A and B). She has palpable right brachial and radial pulses but no palpable left upper extremity pulses. The left hand is slightly cooler than the right. She denies extremity swelling, dizziness, or visual disturbances.
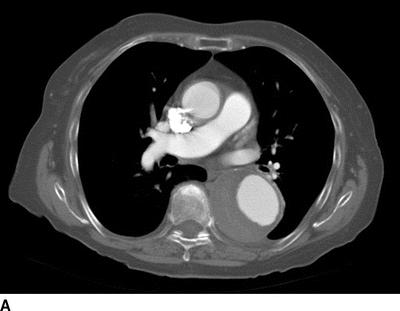
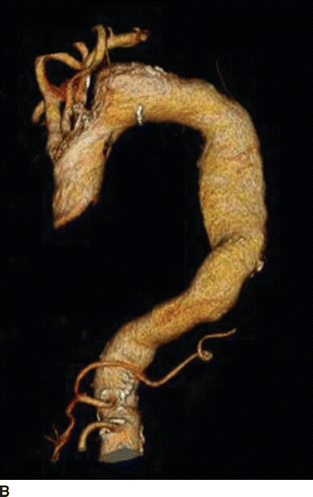
FIGURE 1 A: Preoperative axial CT showing 6.0-cm symptomatic aneurysm of the descending thoracic aorta. B: Preoperative 3D reconstruction showing both saccular and fusiform aneurysms with relationship to LSA.
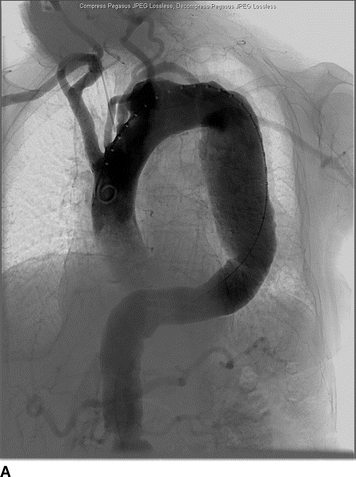
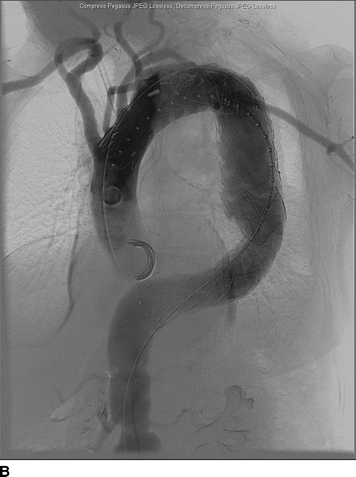
FIGURE 2 A: Initial intraoperative angiogram showing proximity of descending thoracic aortic aneurysm to the LSA. B: Completion angiogram after stent graft deployment with uncovered stents over the origin of the left common carotid artery. The graft material covers the origin of the LSA with retrograde filling from the left vertebral artery.
Differential Diagnosis
Coverage of the LSA during TEVAR can cause symptoms of left upper extremity ischemia, such as pain or numbness. In the absence of symptoms, a pulseless left upper extremity does not indicate ischemia by itself. Pulse deficits are common after subclavian artery coverage, but ischemic symptoms are infrequent. Other causes of extremity pain and paresthesia include nerve injury, extremity arterial occlusive disease, and deep vein thrombosis.
Workup
Carotid duplex ultrasound is recommended in older patients to detect asymptomatic disease of the carotid bulb and internal carotid artery since repair may involve carotid artery clamping. Reversal of flow may be noted in the left vertebral artery. Wrist-brachial indices will be diminished, and segmental arterial waveforms will show attenuation of amplitudes (Fig. 3A and B).
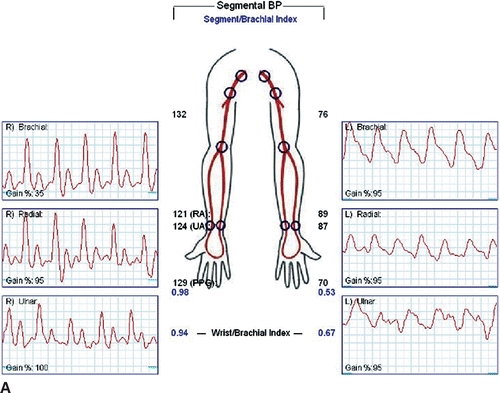
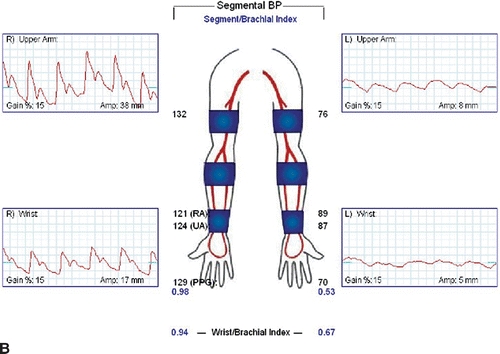
FIGURE 3 A: Wrist-brachial index, segmental waveforms. B: Pulse volume recordings after TEVAR with coverage of the LSA.
It is prudent to review the patient’s pre-TEVAR imaging to insure that there were no missed injuries or additional arterial occlusive disease, such as vertebral, carotid, or subclavian artery dissection or stenoses. If the patient is due for TEVAR surveillance imaging, the chest CTA will typically include enough of the neck and extremity to visualize the proximal to midcarotid and subclavian arteries. If there is doubt about a missed extremity injury or stenoses, dedicated extremity imaging should be obtained. Swelling in the extremity should prompt venous duplex imaging.
Diagnosis and Treatment
The Society for Vascular Surgery consensus guidelines and many authors recommend preoperative subclavian revascularization when coverage is required in elective TEVAR. Preemptive subclavian revascularization may be associated with lower rates of stroke and spinal cord ischemia. Definitive evidence is difficult to obtain since spinal cord injury occurs in less than 5% of TEVAR procedures. In emergent or urgent cases, such as with traumatic aortic injury, acute complicated aortic dissection, or ruptured aortic aneurysms, preemptive subclavian revascularization is not required. The diagnosis of extremity ischemia after LSA coverage with TEVAR is primarily based on history and clinical exam. Postoperative patients who are asymptomatic, even with flow reversal in the vertebral artery, do not require subclavian revascularization.
The most common method of proximal LSA revascularization is carotid-subclavian artery bypass. Other options include subclavian-carotid artery transposition and laser fenestration of the aortic endograft. With the development of commercial branched thoracic aortic endografts, routine subclavian revascularization will become integrated within the initial endovascular procedure.
Case Continued
Noninvasive imaging, history, and physical exam findings are consistent with left upper extremity ischemia after coverage of the LSA with a thoracic aortic endograft. Revascularization options were discussed with the patient, and the decision was made to proceed with left subclavian-carotid artery transposition.
Surgical Approach
Carotid-subclavian bypass using prosthetic graft is simpler and surgically expedient since it does not require as deep a dissection into the mediastinum. Bypass also preserves flow to the left internal mammary artery since the subclavian artery is clamped distal to the vertebral artery and is a consideration for patients with patent coronary artery bypasses. Transposition requires subclavian clamping proximal to the vertebral and mammary arteries. We prefer bypass when the right vertebral artery is occluded or atretic or if the left vertebral artery is not contiguous with the basilar artery. Patency rates slightly favor transposition over bypass. Five-year patency rates approach 98% for transpositions and 88% for bypass. Complication rates are low in both cases.
The patient is positioned supine with a shoulder roll. The left arm should be tucked at the patient’s side. This elevates the subclavian artery superiorly. The neck is slightly extended and rotated to the right.
For a subclavian-carotid transposition, a transverse supraclavicular incision is centered over the sternocleidomastoid muscle. The sternal and clavicular heads are divided at the clavicle and retracted cranially. The omohyoid muscle is divided at the lateral border of the internal jugular vein and retracted laterally. The contents of the carotid sheath are then dissected free; the left internal jugular vein is dissected and retracted laterally; the left common carotid artery is circumferentially dissected; the vagus nerve is identified and protected from excessive retraction. The thoracic duct is ligated with fine silk suture at the junction of the internal jugular and subclavian veins. The thoracic duct travels medially from the mediastinum transversely across the neck, anterior to the vertebral artery and superior to the subclavian artery, to enter the confluence of the internal jugular and subclavian veins. The vertebral vein is ligated. The subclavian artery is dissected proximally into the mediastinum and encircled with a vessel loop. Anticoagulation is begun, and the subclavian, vertebral, and internal mammary arteries are clamped. The proximal subclavian artery is ligated with running 4-0 polypropylene suture. The left common carotid artery is clamped, and a longitudinal posterolateral arteriotomy is made for an end-to-side anastomosis. Shunting is cumbersome and usually not needed since the external carotid artery is not clamped. The arteries are allowed to back-bleed before completing the anastomosis and the clamps are released (Fig. 4A and B).
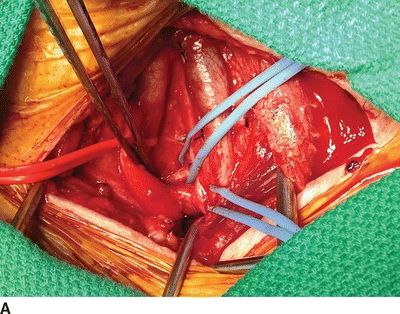
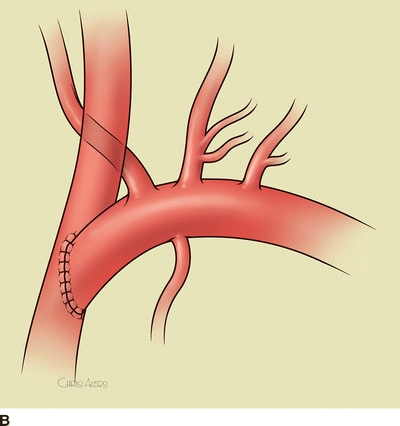
FIGURE 4 A: Intraoperative image of completed subclavian-carotid transposition. The left common carotid artery is retracted medially (red vessel loop), and the vertebral and internal mammary arteries are encircled with blue vessel loops. B: Schematic image of transposition with internal jugular vein removed.



