8
Management of acute lower limb ischaemia
Introduction
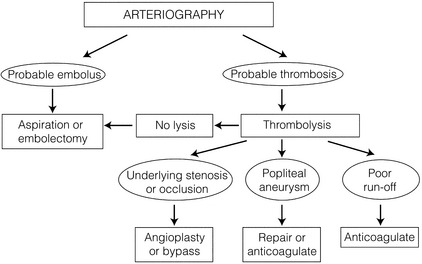
Presentation is usually less than 2 weeks’ duration. However, some overlap with chronic critical leg ischaemia is inevitable. The severity of ischaemia is best defined according to the SVS/ISCVS guidelines (Table 8.1),2 which group patients into the following categories:
Table 8.1
Suggested classification of acute limb ischaemia
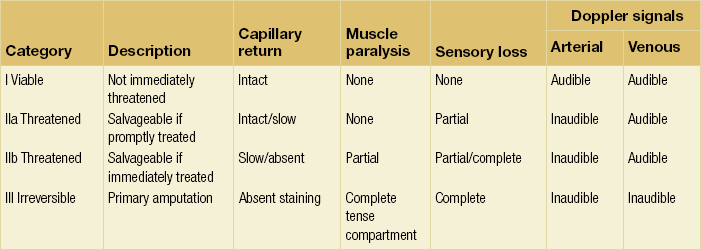
Reprinted from Rutherford RB, Flanigan DP, Gupta SK et al. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg 1986; 4:80–94, with permission from Society for Vascular Surgery.
• IIa Threatened (salvageable if promptly treated).
• IIb Threatened (salvageable if immediately treated).
Data on the incidence of acute limb ischaemia are sparse. Epidemiological surveys would suggest that the incidence is in the region of 140 per million of the general population and this figure appears to be increasing.1 There is evidence that the outcome is improved when patients are managed by a vascular service providing 24-hour cover.3 ALI is associated with a high cost to the community because of the risk of amputation (10–30% at 30 days) and prolonged hospitalisation. Costs are minimised and outcome optimised by accurate clinical assessment and an understanding of the available therapeutic options.
Aetiology
ALI is the result of occlusion of a native artery or vascular/endovascular prosthesis. In situ thrombosis or embolism can cause native arterial occlusion (Box 8.1).
Embolism
Until about 30 years ago, embolism was the underlying cause of most cases of ALI. Emboli large enough to occlude major vessels usually arise in the heart. Rheumatic mitral valve disease was the most common cause, with large emboli forming in a dilated left atrium. Atrial fibrillation due to ischaemic heart disease is now the cardiac origin in 80% of embolic cases; mural thrombus following acute myocardial infarction causes most of the remainder.4 Less commonly, embolisations from mural thrombi of the aorta, aortic aneurysms and iliac arteries are observed. Large emboli typically lodge at an arterial bifurcation, particularly in the common femoral or popliteal arteries (Fig. 8.1). Patients with cardiac embolism may also suffer from peripheral vascular disease as a result of the underlying process of atherosclerosis. This increases the difficulty in establishing the cause of the ischaemia and in planning revascularisation. In 20% of patients with ALI, a source for the embolus cannot be found.
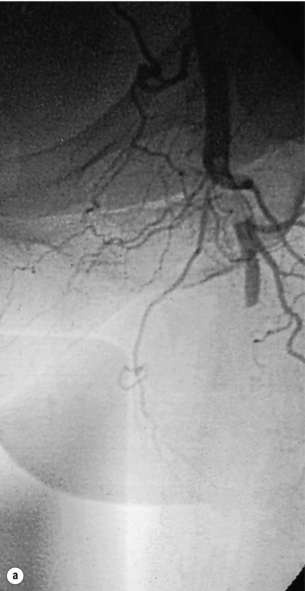
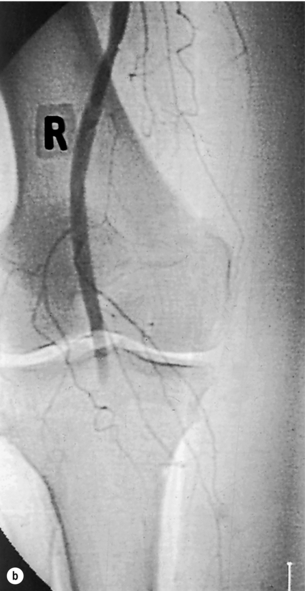
Figure 8.1 Arteriogram demonstrating an embolus lodged in the bifurcation of the common femoral artery (a) with further emboli occluding the distal profunda and popliteal artery (b).
Atheroembolism
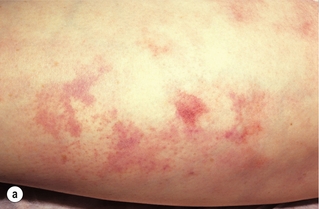
Less common sources of emboli include proximal aneurysms or atherosclerotic plaques, usually located in the thoracic or abdominal aorta. Whereas cardiac embolism usually consists entirely of platelet thrombus, embolism from proximal arteries can include atherosclerotic plaques or cholesterol-rich emboli. This has a much worse prognosis than cardiac embolism because embolectomy is less effective. Small particles of atheroembolism can pass to very distal vessels in the foot. This digital embolism can result in the ‘acute blue toe syndrome’. In this condition, the embolic source should be identified and treated if possible. Often this is a proximal arterial plaque that has ruptured and the emboli are a mixture of platelet thrombus and cholesterol (Fig. 8.2). Embolisation of cholesterol-rich atheroma can occur spontaneously, but also follows intravascular manipulation by endovascular intervention, or occasionally surgery (trash foot). This can be disastrous, since both large and small arteries are occluded and cannot be reopened with either surgery or thrombolysis. This often results in limb or end-organ damage, and is sometimes fatal (Fig. 8.3).
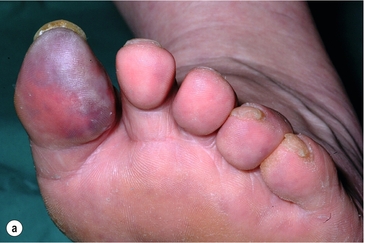
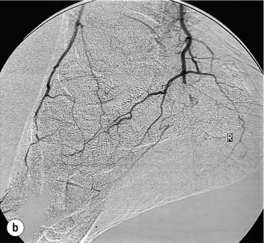
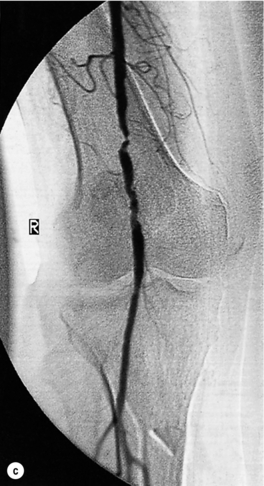
Figure 8.2 (a) ‘Blue toe syndrome’ due to digital and pedal arterial atheroembolism (b) from a proximal atherosclerotic stenosis (c). Note the acute cut-off of the posterior tibial artery. Ultrasonography excluded a popliteal aneurysm and the lesion was treated by balloon angioplasty.
Other causes
The increasing use of both open and endovascular techniques to revascularise ischaemic limbs means that surgeons often have to deal with acute thrombosis of bypass grafts and arteries that have previously undergone endovascular treatment. Grafts occlude for a variety of reasons. Graft occlusion within 1 month of insertion is usually the result of technical problems at the time of surgery or poor distal run-off. Graft occlusion within 1 year of placement is often caused by myointimal hyperplasia at an anastomosis or the development of stenoses within a vein graft. Occlusion after 1 year is usually due to progression of distal atherosclerosis. Prosthetic grafts have a higher occlusion rate than autogenous vein grafts (see Chapters 3 and 7).
Iliac limb occlusions are observed after aortobifemoral bypass surgery if limbs become kinked or have poor outflow and after for similar reasons endovascular stent grafts are used to repair abdominal aortic aneurysms. Occlusion is more common when the iliac limb of the stent graft is extended in to the external iliac artery. Occlusion may occur any time after implantation in up to 5% of patients.5
Recent changes
A number of factors may have changed the presentation of ALI. For example, many patients are now treated with risk factor-modifying drugs such as antiplatelet therapy and statins. Antiplatelet therapy probably reduces the risk of limb deterioration and the need for limb revascularisation in those patients with established peripheral arterial disease (PAD).6 It is not yet clear whether this has had an effect on the incidence of ALI. Similarly, patients with atrial fibrillation are currently treated with prophylactic warfarin to prevent stroke according to their CHADS(2) risk score.7 Whilst this does not entirely prevent peripheral embolisation, it can make the acute ischaemia easier to deal with, since there is less secondary thrombosis, particularly affecting small distal arteries.
Clinical features
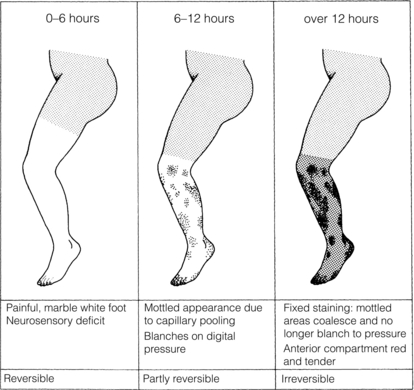
The severity of ischaemia at presentation is the most important factor affecting outcome of the leg.8,9 Complete occlusion of a proximal artery in the absence of preformed collateral vessels (as in cardiac embolism) results in the classical clinical presentation of pain, paralysis, paraesthesia, pallor, pulselessness and a perishingly cold leg. The pain is severe and frequently resistant to analgesia. Calf pain and tenderness with a tense muscle compartment indicates severe muscle ischaemia or necrosis and often irreversible ischaemia. Sensorimotor deficit including muscle paralysis and paraesthesia is indicative of muscle and nerve ischaemia with the potential for salvage if treated promptly. Initially the leg is white with empty veins but after 6–12 hours vasodilatation occurs, probably caused by hypoxia of the smooth muscle. The capillaries then fill with stagnant deoxygenated blood, resulting in a mottled appearance that blanches on digital pressure (Fig. 8.4). If flow is not restored rapidly, the arteries distal to the occlusion fill with propagated thrombus and the capillaries rupture, resulting in a fixed blue staining of the skin that is a sign of irreversible ischaemia. These features are typical of an acute arterial occlusion in the absence of existing collaterals and suggest an embolic cause. When arterial occlusion occurs as part of a chronic process where collaterals have developed, typically the leg is less severely ischaemic. Patients with peripheral atherosclerosis deteriorate in a stepwise fashion as thrombosis supervenes on an existing arterial plaque. Patients often report a sudden change in symptoms, which progress over a few days: the foot often has a dusky hue with slow capillary return. Previous claudication or absent pulses in the contralateral foot help make a clinical diagnosis of in situ thrombosis. Palpation of a mass in either popliteal fossa suggests thrombosis of a popliteal aneurysm. Young patients (<50 years), those with an atypical history (e.g. severe back pain associated with aortic dissection) or recent endovascular intervention raise the possibility of non-atherosclerotic/embolic ALI.
Initial management
Patients presenting with ALI are often in poor general health, which contributes to the observed high mortality rate from associated cardiovascular disease. Dehydration, cardiac failure, hypoxia and pain should all be managed in the standard way. An intravenous infusion is required for rehydration and is also often the best means of providing analgesia with an infusion pump. If thrombolysis is an option, intramuscular analgesia should be avoided because of the risk of bleeding. Intravenous calcium heparin (5000 units) should be given immediately, followed by systemic heparinisation, principally to restrict propagation of thrombus, although there is also evidence that it improves the prognosis.10 In many units low-molecular-weight heparins have replaced calcium heparin because of their more reliable effect on anticoagulation. Anticoagulation should be delayed if the patient is likely to need epidural anaesthesia. The short half-life of the latter, however, is helpful in this situation. In order to improve oxygenation, 24% oxygen should be given by face mask.
Revascularisation
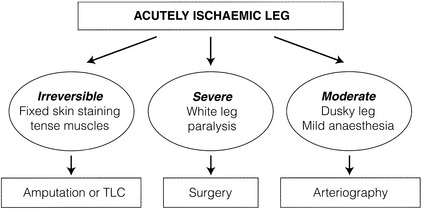
The clinical assessment of the severity of limb ischaemia will largely dictate the most appropriate form of therapy (Fig. 8.5).
Irreversible (category III) leg ischaemia
A small number of patients will present in a moribund state or with irreversible leg ischaemia (muscle paralysis, tense swollen fascial compartments, fixed skin staining) and terminal care should be considered. For the irreversibly ischaemic leg, revascularisation is, by definition, inappropriate and may be dangerous. This includes the patient who develops ALI while being treated for another condition, usually as an inpatient on an elderly care ward. Prognosis is particularly dismal in this group.11 Surviving patients should be resuscitated and stabilised before considering amputation.
Immediately threatened (category IIb ischaemia)
Both groins and lower limbs should be prepared in theatre. If inflow cannot be re-established into the groin then a femoral crossover graft can be inserted (or aortic dissection managed according to Chapter 9).
Threatened (category IIa ischaemia)/viable (category I ischaemia)
The majority of patients presenting with ALI have acute onset of rest pain but no paralysis and no, or only mild, sensory loss. The cause is often acute thrombosis of either an atherosclerotic artery or graft. Because the leg is not immediately threatened, time is available to plan appropriate intervention after investigation. Conventionally, acute leg ischaemia was investigated using catheter (Fig. 8.6). The advantage was that imaging could be followed by therapeutic thrombolysis at the same sitting. There are now, however, non-invasive alternatives such as duplex imaging CT and MR angiography, both of which can provide enough information on which to plan intervention. The method of investigation will depend on the time of presentation and the available facilities. The two main alternatives in these patients are intervention with surgery or percutaneous thrombolysis. Thromboembolectomy is unlikely to reopen an artery occluded by thrombus and atherosclerotic plaque; formal arterial bypass is more likely to be needed. Nationwide registries suggest that surgical revascularisation is used three to five times more frequently than thrombolysis in everyday clinical practice for patients with ALI.12 Logistics (multiple angiographies) and experience may account in part for this distribution.
Some patients are admitted to hospital with acute thrombosis of a peripheral artery (usually the superficial femoral) but have only claudication and no ischaemic pain at rest. Thrombolysis might appear an attractive option to treat the claudication, but the risks are as high as in patients with limb-threatening ischaemia.13 Initial anticoagulation followed by expectant management, depending on the progression of ischaemia, reduces the risk to both life and limb.10 Some patients’ symptoms improve and never require revascularisation. In others (especially those with short-distance claudication) it may be more appropriate to angioplasty the occluded arterial segment 6–12 weeks after the acute event, when the thrombus has organised and embolic risk is reduced.
Choice between surgery and thrombolysis: the evidence
There remains considerable controversy about the individual roles of surgery and thrombolysis for ALI. A review of 42 studies (most non-randomised) by Diffin and Kandarpa14 suggested that thrombolysis was associated with substantially better limb salvage and mortality rates than surgery.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree