In the United States, abdominal aortic aneurysm (AAA) is ranked as the 13th leading cause of death and is largely related to the high mortality rate associated with aneurysm rupture (up to 80%). The success rate in treating an intact AAA, however, is over 95%, which underpins the objective of early detection and treatment of AAA before rupture. Until recently the only treatment modality available was open surgical repair, which required general anesthesia and open laparotomy. The first successful surgical repair of an AAA was performed by Dubost et al. in 1952 (1). Since then, significant improvements related to surgical repair of AAAs have been made. Current studies report an impressive surgical mortality rate of 2.1% to 5.8% following open elective repair (2–6). However, it is important to note that there are other risks besides mortality in performing a major laparotomy. These include high rates of major complications including prolonged hospitalization, prolonged recuperation, and sexual dysfunction (7). Moreover, there are patients who are not surgical candidates, owing to comorbid conditions. As a result, there was a significant unmet need for a less invasive approach to treat patients with AAAs. This led to the development of endovascular aneurysmal repair (EVAR), which was first performed and reported by Juan Parodi in the early 1990s. EVAR can accomplish AAA repair without the need for major laparotomy and/or general anesthesia. Since that time, significant improvements in both technique and devices have been made. Currently, five endografts are approved by the Food and Drug Administration (FDA) for EVAR.
This chapter provides a description of the general indications for surgical repair of AAA, the specifi c indications for EVAR, the various types of endovascular grafts that are currently available for AAA, and the principal techniques used to achieve EVAR.
GENERAL INDICATION FOR AAA THERAPY
At the present time, the same size (i.e., maximal aortic diameter) criteria for open repair should be used for EVAR, as there are no data to justify performing EVAR for smaller aneurysms. In 1966, the first paper discussing the size of AAA as an indication for open repair was published. Measurement on physical examination of more than 6.0 cm was used as a cut off to proceed with the repair. Since then, extensive studies have been performed to defi ne the standard for management.
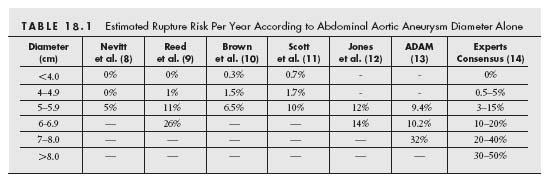
There appears to be a consensus opinion, based on estimates of rupture risk with increasing aortic diameter (Table. 18.1), that AAA >5.5 cm should be repaired. Because of this consensus, no randomized controlled trials (RCT) have been performed in this patient cohort.
Although size is a very important factor in predicting AAA rupture risk, there are other factors that appear to play an important role. Each of the following has been found to be an independent risk factor for AAA rupture (3,15,16):
- chronic obstructive pulmonary disease (COPD);
- hypertension (HTN);
- female gender;
- smoking;
- symptoms: abdominal or back pain.
Other nonindependent risk factors include:
- First-degree relatives with AAA (15% to 35% increase rupture risk based on the number of first-degree relatives).
- The ratio of the AAA diameter to the native aorta diameter.
- The shape of the aneurysm. Saccular aneurysms have a higher risk than fusiform aneurysm. This is probably related to the significant increase in wall stress with eccentric aneurysms leading to rapid expansion and rupture.
- The degree of expansion. It continues to be regarded as a significant risk factor for rupture. Aneurysms that enlarge more than 0.6 cm in diameter in 1 year are at a higher risk for rupture, and thus require earlier repair. Smoking, HTN, and mural thrombus are each associated with rapid expansion.
Given the uncertainty regarding the optimal management of patients with aneurysms less than 5.5 cm in diameter, two prospective randomized multicenter studies have been performed in this patient cohort: the United Kingdom Small Aneurysm Trial (UKSAT) (2) and the Aneurysm Detection and Management trial (ADAM trial) (3). Each study prospec-tively randomized patients (n = 1090 and 1136, respectively) with asymptomatic AAAs between 4.0 and 5.5 cm in diameter to undergo early elective open surgery (early surgery group, n = 563 and 569, respectively) or surveillance with ultrasonography or computed tomography (CT) scan, every 3 to 6 months (surveillance group, n = 527 and 567, respectively). The mean follow-up period for the entire group was 4.6 and 4.9 years in the UKSAT and ADAM trials, respectively. If the diameter of an AAA exceeded 5.5 cm, the AAA expanded by more than 1.0 cm in 1 year, or the patient became symptomatic, surgical repair was recommended. The primary end point was death, and analysis was performed by intention-to-treat.
Both groups had similar cardiovascular risk factors at baseline. At the end of the trial, surgical repair was performed in 90% of the patients in the early surgery groups, and in 60% in the surveillance groups of both studies. Mortality rates did not differ significantly between the two groups at 2, 4, or 6 years. However, the 30-day operative mortality in the early surgery group for the UKSAT was 5.8%, which led to a survival disadvantage early in the trial. In the ADAM trial, the immediate post-operative mortality was significantly lower at 2.7%, but the overall rate of aneurysm-related death in the early surgery group was similar to that in the surveillance group (3.0% vs. 2.6%). Age, sex, or initial aneurysm size did not modify the overall hazard ratio. The conclusion for both studies was that radiographic surveillance with delayed surgery for small AAAs (4.0 to 5.5 cm) is safe, and immediate surgery does not provide a long-term survival advantage.
As a result of these recent findings, the Society for Vascular Surgery Guidelines for the treatment of AAA have been modified. The previous guidelines recommended surgical treatment for an AAA greater than 4.0 cm in diameter. This size threshold has been modified to 5.5 cm (14).
EVAR VERSUS OPEN REPAIR
Short-term Outcome
Intuitively, EVAR seemed to be safer compared with open repair owing to its less invasive nature. However, the EVAR-1 (5) and DREAM trials (4), both of which were randomized trials comparing EVAR and open repair, were pivotal in assessing relative performance of EVAR versus conventional open surgical repair. Both trials randomized patients with AAA that were suitable for both EVAR and open repair. Both trials showed a strikingly similar result, with a one-quarter to one-third reduction in 30-day mortality following EVAR compared with open surgery (EVAR-1: 1.7% vs. 4.8%, DREAM: 1.2% vs. 4.6%, both statistically significant). During the follow-up period, there were many cases of unrelated deaths due to cardiac and pulmonary causes, and death due to cancer. As a result, the overall survival rate was similar in both groups. However, even at 4 years following treatment, the EVAR patients enjoyed a 3% higher freedom from aneurysm-related death, which was unaffected by the aforementioned background noise (i.e., non-aneurysm-related death) (5).
In addition to operative mortality, patients undergoing open repair require several weeks for recovery. Approximately 20% to 30% of patients suffer from major operative complications including pneumonia, myocardial infarction, intestinal obstruction, bleeding, renal infarction, and peripheral embolism (17,18). Williamson et al. analyzed 139 patients who underwent open repair and found that 36% of the patients were non-ambulatory post-operatively, and 11% required transfer to a nursing home for 4 months or longer post-operatively (18). Furthermore, 80% of patients had post-operative sexual dysfunction.
Based on these favorable results as well as those obtained from observational studies (19), EVAR has become the “default” standard therapy for the treatment of AAA, assuming the anatomy is suitable for EVAR. In fact, it is estimated that more than half of the 60,000 AAA treatments performed per year in the United States are performed with EVAR (17).
Mid-/Long-Term Results
In terms of long-term outcome, open repair has been studied quite extensively owing to its longstanding availability. Although its long-term outcome is very acceptable, it is noteworthy that open repair has not been subjected to the same level of scrutiny that EVAR has been subjected to, and therefore it is highly likely that the late complication rate following open repair is underestimated. For example, in a recent study by Conrad et al., only 57% of the 269 open cases underwent follow-up radiologic studies (i.e., CT or magnetic resonance imaging [MRI]) (20). Keeping this in mind, the reported need for secondary procedures following open repair ranges between 3% and 15%, during 5 to 10 years follow-up (20–22). In order to assure accuracy and minimize the “lost to follow-up” rate, Hallett et al. conducted a survey including only those patients who lived near the hospital (21). This study reported that 9.4% of patients had complications related to open repair or the aortic graft during a mean follow-up period of 5.8 years. Late complications following open repair include graft infection, para-anastomotic aneurysms, aortoenteric fi stula, graft thrombosis, ileus, and incisional hernia (20–22). Although the incidence of such complications may not be alarmingly high, once they occur, the operation for treating them is often difficult, and the mortality rate accompanying a secondary operation has been reported to be about 15%.
Durability has been considered the major limitation of EVAR. In fact, high rates of secondary intervention have been reported. However, it is also true that these poor outcomes were mainly based on studies that utilized either homemade or first-generation endografts. Recent studies have shown that the current generation endografts perform better than these early generation devices. As shown in the EVAR-1 and DREAM trials that utilized second-generation endografts, treatment outcomes have improved. Other evidence comes from the FDA-approved Excluder clinical trial (W.L. Gore & Associates, Flagstaff, AZ) that was conducted in the United States. At 5 years follow-up, there were no ruptures in either the open repair group (99 cases) or the EVAR group (235 cases). As for the rate of major adverse events, the EVAR group had a sustained benefi t at 5 years compared with the open repair group. In addition, stent migration (clinically relevant), stent fracture, and limb thrombosis were not seen at all.
One unique aspect of late failures following EVAR includes the fact that secondary intervention can almost always be performed with catheter-based techniques and therefore is very safe and minimally invasive (4,5,23).
The operative mortality for open repair is significantly higher if one of the following factors is present (Canadian Aneurysm Study (24), UKSAT (2)):
- electrocardiographic changes indicating ischemia;
- congestive heart failure;
- COPD with forced expiratory volume in 1 minute (% FEV 1.0 L) of less than 70%;
- elevated serum creatinine greater than 1.8 mg/dL;
- age older than 75 years.
The 30-day operative mortality may range between 2% and 50%, based on the number of the above factors present. The presence or absence of these risk factors might be helpful in selecting the appropriate treatment modality.
Morbidity rates are significantly lower with EVAR. The relative reduction in complication rates approaches 30% to 70% (14), and this might be attributed to the significant reduction in anesthesia time, blood loss, and fewer cardiac complications. The reduced invasiveness (i.e., avoidance of the laparotomy and exposure of the retroperitoneal cavity) and smaller incisions (usually limited to groin incisions for access) associated with EVAR result in significantly less gastrointestinal complications and less post-operative pain, allowing early ambulation and oral intake. Reducing anesthetic time, incision pain, and early am-bulation ultimately reduces post-procedural pulmonary complications. The end result is shorter hospital stays (6,25). The most striking difference between EVAR and open repair is the remarkable difference in recovery time. Up to 30% of patients with open repair did not recover at 34 months postoperatively, and 18% of patients indicated that they would not have open repair again knowing the recovery process. Sexual dysfunction following open repair approaches 60% to 80% in some studies (7). This is significantly less with EVAR because there is no dissection of pelvic nerves. Even in the case of intentional coverage of the internal iliac arteries, the incidence of post-operative sexual dysfunction is less than 1% with EVAR. Early conversion of EVAR to open surgery has significantly decreased from 10% to 2%, as patient selection has improved, devices have become more sophisticated, and surgeons/interventionalists have become more experienced. Reported late conversion rates are now reported at 1% to 2% per year. Typical causes of late conversion include aneurysm enlargement, device migration, structural graft failure, and late rupture of the aneurysm (23,26). Secondary conversion is associated with higher morbidity and mortality rates. All of these factors make open repair ideal for younger patients who are less likely to have high-risk characteristics for open repair. Bush et al. studied patients who underwent EVAR but were deemed high risk for open repair (27). They found that outcomes after EVAR were excellent and that the procedure is safe even in high risk patients. Patient choice remains an important factor in selecting the treatment modality (14).
INDICATIONS FOR TREATMENT OF AAA USING EVAR
Since its first introduction in 1991, EVAR has gained significant acceptance (28,29). However, not all patients are anatomically suitable for EVAR. The critical anatomic elements that determine the suitability of a patient for EVAR include (Fig. 18.1):
- Patent superior mesenteric artery (SMA) or celiac trunk.
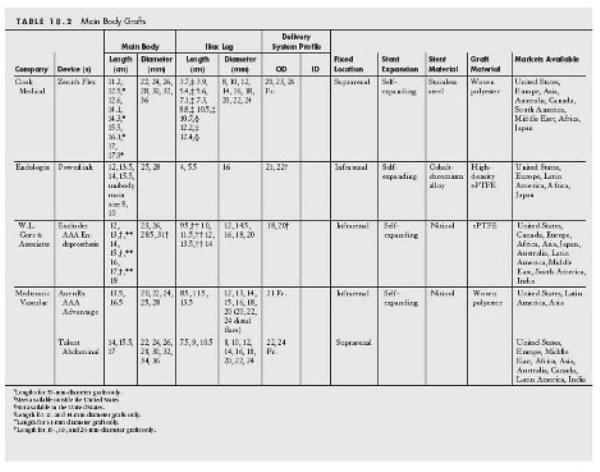
- An infrarenal neck diameter of 32 mm. This is based on the fact that the graft is typically oversized by 10% to 20%, and the largest graft available has a 36-mm diameter (Cook, Zenith; see Table. 18.2).
- The length of the proximal neck has to be at least 10 to 15 mm, to allow for a proper seal of the stent graft against the aortic wall.
- Neck angulation of less than 45 to 60 degrees, since this is associated with a lower incidence of type 1 endoleaks.
- Calcifi cation and mural thrombus encompassing more than 90 degrees of the circumference of proximal neck is unfavorable (Fig. 18.2).
- A contour change of the proximal neck of more than 10% is associated with a higher incidence of type 1 endoleaks (i.e., flared neck).
- The minimal diameter of the external iliac artery should be 6 to 7 mm to allow the passage of the device. The smallest caliber delivery system for the main body of a stent graft in the US market is 18 Fr. (Excluder, W.L. Gore).
- Distal fixation requires a 10- to 15-mm length of normal vessel in the iliac segment, similar to that required for proximal fixation.
- The diameter of the common iliac artery (CIA) should be less than 20 mm. If the CIA diameter is greater than 20 mm, an additional cuff is required to extend the stent graft into the external iliac artery following coiling of the internal iliac artery.
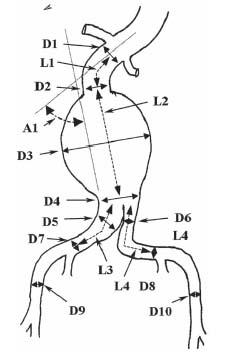
Figure 18.1 • Illustration of diameter, length, and angle measurements made in each patient with an abdominal aortic aneurysm to assess the patient’s eligibility for EVAR and also to select the appropriate endograft and the appropriate size of each component of the endograft. These are typically obtained using 3D reconstructed CT scan images. Diameters: D1 and D2, proximal neck of aneurysm; D3, aneurysm; D4, distal neck of aneurysm; D5 and D7, right common iliac artery; D6 and D8, left common iliac artery; D9, right external iliac artery; D10, left external iliac artery. Lengths: L1, length of proximal neck; L2, length of aneurysm; L3, length of right iliac limb; L4, length of left iliac limb; A1, neck angle.
These summarize the most common anatomic criteria for a patient to be treated with EVAR. It has been shown that those patients who have unsuitable anatomy do poorly, in both short- and long-term follow-up (30). This is especially true for those who have anatomically unfavorable proximal necks (short, angulated, and wide necks). Thus, we believe that it is of paramount importance to adhere to these anatomic criteria.
PRE-OPERATIVE IMAGING EVALUATION
Computed Tomography
Contrast-enhanced CT is the standard pre-operative imaging modality for EVAR. A high-quality scan can be reconstructed in three dimensions.
The advantages of CT imaging include:
- The ability to evaluate vessel wall calcifi cation and mural thrombus.
- Compared with conventional angiography, it is nonin vasive, utilizing only a peripheral intravenous (IV) to administer contrast.
- It enables accurate measurement of all the dimensions needed to perform the EVAR procedure including the diameter, length, and angle of the proximal neck as well as the iliac vessels. If one can utilize the three-dimensional (3D) CT workstation, these measurements will be more precise. A workstation provides a 3D reconstruction of the CT angiogram, and allows one to rotate the images in the sagittal, coronal, and axial axes, and obtain all measurements required for proper graft selection (Fig. 18.3). An additional advantage is that it provides aneurysmal volume measurements, and it shows the precise location and the presence of disease at the orifice of critical branch vessels (i.e., celiac trunk, SMA, inferior mesenteric artery [IMA], renal arteries, and internal iliac arteries).
The disadvantages of CT include:
- The risk of contrast-induced allergic reaction (1% to 2%).
- The risk of contrast-induced nephropathy.
- Pitfalls of sizing by CT angiography. A major pitfall of axial imaging using CT is that aortic neck deviation may not be taken into account. As the vessel enlarges, it also elongates, resulting in tortuosity and deviation. This may result in over-estimation of the neck diameter (i.e., when the aortic lumen is oval rather than round) and under-estimation of the neck length. This may be overcome by image processing with curvilinear reformatting, perpendicular to an imaginary line inserted in the center of the vessel lumen. If reformatting is not available, one may estimate the angulation and factor in the extra length. In an effort to guard against an over-estimation of the neck diameter, the minor diameter of the neck should always be used. The authors utilize the Aquarius NetStation (TeraRecon, Inc., Powering Imaging Innovation) to make precise measurements of the length of the neck. This workstation allows simultaneous presentation of 3D volume rendering, multiplanar reconstruction (MPR), maximum intensity projection (MIP), minimum intensity projection (MinIP), RaySum, thick MPR, perspective 3D, and Curved Planar Reformat (CPR) data from the arterial segment of interest. CPR is particularly useful in making measurements of the length of proximal neck, the length from the lower renal artery to the internal iliac artery, and the length of distal neck (Fig. 18.3).
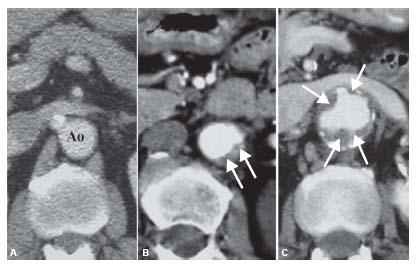
Figure 18.2 • Quality of the proximal neck. A: Ideal proximal neck with absence of plaque or mural thrombus (Ao, aorta). B: Acceptable neck for EVAR with thrombus (arrows) occupying 90 degrees of the aortic circumference. C: Illustration of extensive mural thrombus in proximal neck (arrows), which is a contraindication for EVAR.
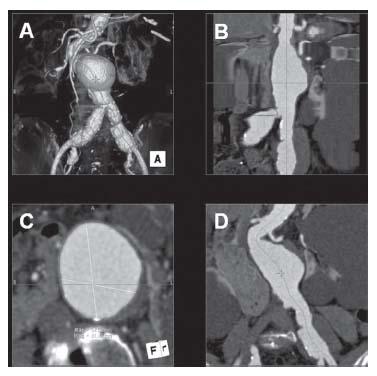
Figure 18.3 • Example of 3D reconstruction of an abdominal aortic aneurysm. A: 3D reconstruction image. B: Curved Planar Reformat (CPR) presents the abdominal aorta in a straight line, which allows for calculation of the precise length of proximal neck and distal neck. C: Axial image. D: Multiplanar reconstruction (MPR) image.
Conventional Angiography
In the vast majority of cases, high-resolution CT scan imaging should be suffi cient for thorough evaluation of the anatomy of the AAA, and for accurate graft selection. On rare occasions, preoperative angiography may be benefi cial. However, this can always be performed at the time of EVAR. Pre-operative angiography may be benefi cial for beginner interventionists.
The advantages of conventional aortography include better evaluation of aortoiliac occlusive disease and major branch stenosis, including main and accessory renal and mesenteric arteries. However, angiography is not as good as CTA for evaluating vessel calcifi cation, and may not be able to measure the neck diameter accurately, owing to the inability to detect thrombus within the neck.
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) is an alternative diagnostic modality to CT angiography, with the singular advantage of eliminating the use of ionizing radiation.
The disadvantages of MRA include:
- MRA is inferior to CT scan in terms of spinal resolution.
- In 10% to 15% of patients, MRA may not be performed, owing to metal implants that produce artifacts, or patient intolerance due to claustrophobia.
- MRA is more expensive than CTA.
- MRA does not demonstrate vessel wall calcifi cation.
Ultrasound is useful mainly for screening purposes, as it is readily available and is relatively inexpensive. However, it does not provide sufficiently accurate information to allow measurement of critical vessel lengths, diameters, and angulation. It is also highly operator-dependent.
PRE-OPERATIVE CLINIAL EVALUATION
Although EVAR may be performed safely under local or regional anesthesia, one may not be sure that it will be successful. If difficulties are encountered during the course of EVAR, one needs to decide whether to proceed or to discontinue and convert to an open repair. Without knowing the risk of open repair in the individual patient, a sound decision regarding conversion may not be made, and therefore, it is important to perform a thorough pre-operative cardiac and pulmonary evaluation.
Physical Exam
It is important to document the presence or absence of pulsatility in the aneurysm pre-operatively. Once EVAR is performed, an abdominal examination should be performed and compared with the pre-operative exam. This will provide insight into the effectiveness of the aneurysm exclusion. For the same reason, one should document the lower extremity pulse exam prior to any EVAR procedure.
TYPES OF ENDOGRAFTS
The experience with endovascular EVAR had revealed important insights regarding the relationship between endograft design and endograft performance:
- Small, tapered, and trackable delivery systems ≤20 Fr. in diameter) rarely fail to traverse tortuous iliac arteries.
- Transmural barbs provide the most secure means of proximal attachment.
- Modular endografts are more versatile than unibody endografts.
- Fully stent-supported graft limbs are less prone to thrombosis than unstented graft limbs.
- Long-trunk/short-limb systems are more stable than short-trunk/long-limb systems.
- Low-porosity or permeability fabric is associated with lower rates of aneurysm dilatation in the absence of an endoleak.
- Unpolished (black) nitinol is prone to fracture.
A number of industry-made devices have been approved by the FDA, and have become available in the United States, including the AneuRx (Medtronic, Inc., Santa Rosa, CA), the Zenith (Cook Medical, Bloomington, IN), the Excluder (W.L. Gore & Associates, Flagstaff, AZ), the Powerlink (Endologix, Inc., Irvine, CA), and the Talent (World Medical Corp./ Medtronic, Inc., Minneapolis, MN) stent grafts (Fig. 18.4).
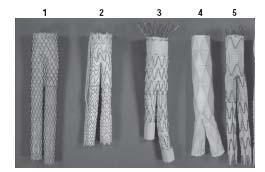
Figure 18.4 • Endovascular grafts (EVGs) approved by the FDA. (1) Medtronic AneuRx, (2) W.L. Gore Excluder, (3) Cook Zenith, (4) Endologix Powerlink, and (5) Medtronic Talent.
DESCRIPTION OF INDIVIDUAL DEVICES APPROVED BY THE FDA FOR EVAR
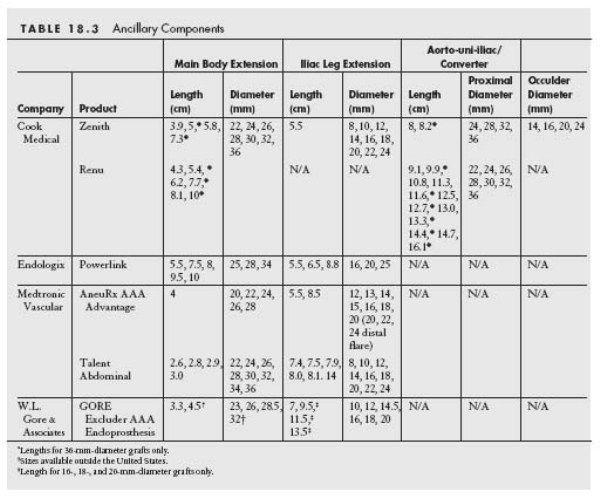
See Tables 18.2 and 18.3.
AneuRx (Medtronic)
The AneuRx was the first modular device available in the United States. This system has a self-expanding nitinol stent as a fixation device and low-porosity Dacron fabric. It is a modular device composed of a main graft and a separate contralateral limb component (Fig. 18.4). The stent is located on the outside of the graft, which is believed to increase the fixation to the native artery. The chief advantage of this device includes its versatility. The company provides a full line of proximal and distal extension cuffs (12 to 28 mm diameter) that may be used to accommodate a wide range of distal landing zone diameters. Following deployment of the main graft, a contralateral limb is inserted, via the contralateral femoral artery, and deployed. In 2004, an enhanced fabric version of the AneuRx was introduced. Additional improvements included the Xpedient delivery system for accurate and easy deployment, the use of high-density Dacron that eliminates type 4 endoleaks, and availability of larger sizes for treatment of aneurysms with larger diameter proximal and distal landing zones. The outer diameters of the main body and contralateral limb delivery systems are 21 Fr. and 16 Fr., respectively. The AneuRx is suitable for treatment of AAA with proximal aortic neck diameters of 20 to 28 mm, and iliac artery diameters of 12 to 16 mm.
Excluder (W.L. Gore)
The Excluder also employs a nitinol stent as a fixation device and polytetrafl uoroethylene (PTFE) fabric. One reason for the difference in sac shrinkage rate following EVAR is the different degree of porosity or permeability of the endograft fabric. The low-permeability Excluder device was introduced in 2004. The additional film layers decreased the overall permeability of the expanded polytetrafl uoroethylene (ePTFE) device. It is a modular device, composed of a main graft and a separate contralateral limb component (Fig. 18.4). The chief advantage of this device is the small crossing profile, with the main body and contra-lateral limb being 18 Fr. and 12 Fr., respectively. This has been extremely helpful in treating patients with diseased and/or small caliber iliac vessels. Because the stent graft is flexible, it can be advanced in cases with an extremely tortuous access route and in cases with significant angulation of the aneurysm neck. In addition, the stent is attached to the graft material with thin layers of PTFE rather than sutures, a feature that is thought to result in less endograft failure. The main graft is constrained by an outer wrap, and is deployed by pulling a rip cord that releases the outer wrap. The Excluder does not have an aorto-uni-iliac (AUI) converter (see Fig. 18.12), but two aortic cuffs of the same size are used in the case of a need to convert to an AUI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree