Malignant Pleural Effusions
A malignant pleural effusion is diagnosed by detecting exfoliated malignant cells in pleural fluid or demonstrating these cells in pleural tissue obtained by percutaneous pleural biopsy, thoracoscopy, thoracotomy, or at autopsy. In a number of patients, even though the pleural effusion is caused by the malignancy, neoplastic cells cannot be detected in pleural fluid or pleural tissue and, in fact, probably are not present in these tissues. It is logical to categorize these pleural effusions associated with malignancy, in which there is no direct pleural involvement with tumor and no other cause for the effusion is found, as paramalignant effusions (Table 77-1). Lymphatic obstruction appears to be the most common mechanism for the development of a paramalignant effusion. Other local effects of the tumor causing a paramalignant effusion are bronchial obstruction resulting in pneumonia or atelectasis. Furthermore, it is important for the clinician to recognize that effusions can result from systemic effects of the tumor and adverse effects of therapy.
The presence of a malignant pleural effusion secondary to lung cancer portends a poor outcome.1 Similarly, a malignant effusion secondary to a nonlung primary is a manifestation of far advanced disease and is associated with limited survival.
MALIGNANCIES ASSOCIATED WITH PLEURAL EFFUSIONS
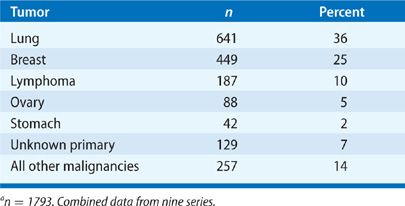
Carcinoma of any organ can metastasize to the pleura. However, carcinoma of the lung is the most common malignancy to invade the pleura and produce malignant and paramalignant effusions (Table 77-2). Carcinoma of the breast is second in incidence and, in some populations, exceeds lung cancer as a cause of malignant effusions. Together, both malignancies account for 65% of all malignant pleural effusions.2 After lung and breast cancer, the frequency declines markedly, with ovarian and gastric cancer representing up to 5% of malignant pleural effusions. Lymphoma accounts for approximately 10% of all malignant pleural effusions and is a common cause of chylothorax. Carcinomas of the lung, breast, ovary, and stomach and lymphomas account for about 80% of all malignant pleural effusions. In approximately 7% of patients with malignant pleural effusions, the primary site is unknown when the diagnosis of a malignant pleural effusion is first established.3
A less common cause of a malignant pleural effusion, other than metastatic carcinoma and lymphoma, is a primary tumor of the pleura, malignant mesothelioma. The association of asbestos exposure and malignant mesothelioma was documented in the 1960s following an initial report from the North Western Cape Province of South Africa and a subsequent study of insulation workers in this country. Owing to the long latency period of 20 to 40 years between exposure and onset of disease, death due to a malignant mesothelioma is expected to reach 9000 annually in Europe and 2200 annually in the United States in 2020.
PATHOGENESIS
Lymphatics are situated beneath the parietal pleura over the intercostal spaces. An important feature of the parietal pleura is lymphatic stomata, 2- to 12-μm openings between parietal pleural mesothelial cells. The stomata and their associated lymphatic channels form lymphatic lacunae immediately beneath the mesothelial layer. These lacunae coalesce into collecting lymphatics, which join the intercostal trunk vessels with flow directed mainly toward the mediastinal lymph nodes. The lymphatic system of the parietal pleura plays a major role in the resorption of pleural liquid and protein. Interference with the integrity of the lymphatic system between the parietal pleura and mediastinal lymph nodes can result in a pleural effusion.4–7 Autopsy series have indicated that impaired lymphatic drainage from the pleural space is the predominant mechanism for the accumulation of fluid associated with malignancy: A strong relationship was found between carcinomatous infiltration of the mediastinal lymph nodes and the occurrence of pleural effusion. In contrast, no relationship was found between the extent of direct pleural involvement by metastasis and the occurrence of a pleural effusion. Further support for this mechanism is provided by the observation that pleural effusions generally do not develop when the pleura is involved by sarcoma because of the characteristic absence of lymphatic metastases.
When pleural metastases develop, tumor cells either “seed” the mesothelial surface or invade the subserous layer: When the mesothelial surface is involved, abundant tumor cells can be found in pleural fluid; with subserous involvement, a paucity of malignant cells are exfoliated into the pleural space. Tumor involvement of the pleura causes reactive changes in the mesothelium that may lead to mesothelial shedding, mesothelial thickening, and, on occasion, marked pleural fibrosis. Pleural fibrosis, usually observed in the more advanced stage of tumor involvement of the pleura, is at least partially responsible for the low concentrations of glucose and the low pH seen in some malignant pleural effusions and for the failure to achieve pleurodesis after instillation of chemical agents.
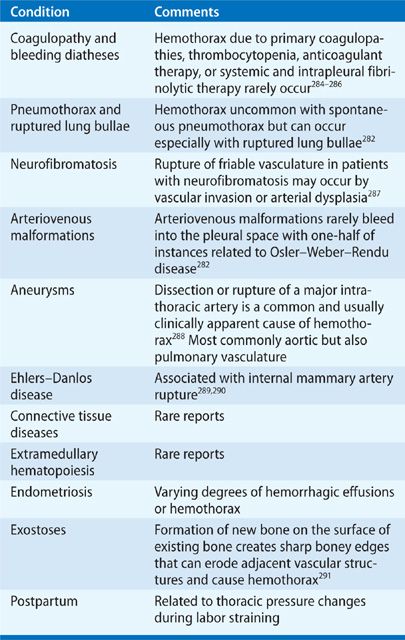
A bloody, malignant pleural effusion usually results from direct invasion of blood vessels, occlusion of venules, tumor-induced angiogenesis, or possibly increased capillary permeability due to vasoactive cytokines and chemokines. Malignant pleural effusions usually contain a large number of morphologically normal lymphocytes, usually in the 50% to 70% range, but typically less than that occurs with tuberculous pleurisy (usually ≥80%). Although the explanation for the lymphocytosis is unclear, these lymphocytes are predominantly T lymphocytes that appear to play a role in the local defense against tumor invasion of the pleural cavity. The percentage of mesothelial cells in malignant effusions is variable, ranging from few to a large percentage of the total cells. An abundance of mesothelial cells occur early in the course of pleural infiltration, before pleural fibrosis and marked infiltration with tumor; in more advanced stages of pleural metastasis, fewer mesothelial cells are generally seen because of pleural fibrosis.
Autopsy findings in patients with malignant effusions have provided valuable information about the pathogenesis of pleural metastases. When carcinoma of the lung metastasizes to the pleura, both the visceral and parietal pleural surfaces tend to be involved. The visceral pleural surface is rarely, and the parietal pleural surface is almost never, the sole site of involvement. Parietal pleural involvement in lung cancer most likely results from neoplastic spread across the pleural cavity from visceral pleural sites along pleural adhesions that are either preformed or secondary to the malignant process.4–7 The pathogenesis of visceral pleural metastasis in lung cancer appears to be through pulmonary artery invasion and embolization.5 The histological type of lung cancer does not appear to determine the propensity for pulmonary arterial invasion. Adenocarcinoma of the lung is the most common cell type to involve the pleura because of its peripheral location and spread by contiguity. Bilateral pleural metastases in lung cancer are almost always associated with hepatic involvement and parenchymal invasion of the contralateral lung.
Pleural metastases from primary sites below the diaphragm generally are a manifestation of tertiary spread from established liver metastases. The data with breast cancer are conflicting; some studies show a high incidence of ipsilateral pleural effusion, while others show no such predilection. Probably two mechanisms are operative, chest wall lymphatic invasion resulting in an ipsilateral effusion and hepatic spread with bilateral or contralateral disease.
At diagnosis, pleural effusions are rare in Hodgkin disease but not infrequent in non-Hodgkin lymphoma. Pleural effusions can be found in previously untreated patients with non-Hodgkin lymphoma, even in the absence of detectable intrathoracic lymphadenopathy; however, the pleural effusion is usually not an isolated manifestation of the disease. At autopsy in Hodgkin disease, lymphomatous infiltration of the lung rather than direct pleural invasion or mediastinal adenopathy has been found in association with the pleural effusion. Lymphomatous invasion of the pleura appears to be an uncommon and late finding in Hodgkin disease but is seen with increased frequency in non-Hodgkin lymphoma. As Hodgkin disease progresses, the incidence of pleural effusion increases and approaches 30%. At autopsy, a 30% to 60% incidence of pleural effusions and a 7% to 30% incidence of pleural nodular infiltrative lesions have been noted.8
While pleural effusion in lymphoma can be due to impaired lymphatic drainage secondary to mediastinal adenopathy, pleural or pulmonary infiltration, or thoracic duct obstruction, impaired lymphatic drainage appears to be the primary mechanism in Hodgkin disease and direct pleural infiltration the predominant cause in non-Hodgkin lymphoma.9
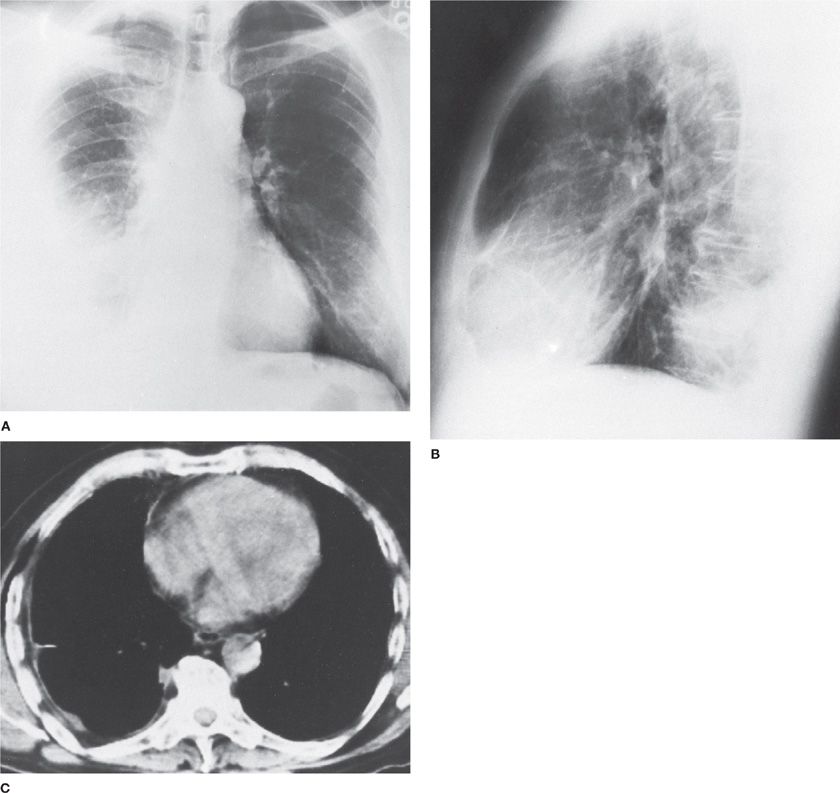
Malignant mesothelioma (see Chapter 79) is usually a unilateral disease (Fig. 77-1); bilateral tumors are present in less than 10% of patients. An early manifestation of the tumor is a pleural effusion that is reabsorbed or organized and then largely replaced by tumor and fibrosis. At autopsy, the lung is often encased in tumor that involves both visceral and parietal pleural surfaces. The pleural space is often obliterated, and the amount of pleural fluid is variable. The tumor seldom penetrates deeply into the lung parenchyma; instead, it extends into interlobar fissures. Hilar lymph nodes are involved by tumor in less than 50% of patients. Distant hematogenous metastases are unusual but have been described in liver, bone, adrenals, thyroid, and kidneys.10–13
Figure 77-1 Malignant mesothelioma in a 64-year-old man. A, B. Diffuse, right-sided involvement. C. Computed tomography scan shows peripheral disposition of mesothelioma along right pleura. The radiodensity in the right hemithorax is a consequence primarily of pleural tumor with little pleural effusion, subsequently treated by right extrapleural pneumonectomy. (Used with permission of Dr. David Murphy.)
The two distinct histological types of malignant mesothelioma (epithelial and sarcomatous) generally behave differently. Some patients have mixed tumors with both epithelioid and sarcomatous features. The clinical features of epithelial mesothelioma are similar to those of metastatic carcinoma of the pleura associated with tumor spread by direct extension, that is, a large pleural effusion and metastases to regional lymph nodes. In contrast, patients with sarcomatous mesotheliomas tend to have features characteristic of sarcomas, that is, distant metastases are common, whereas there is little or no pleural effusion. These data are consistent with the pathogenesis of pleural effusions in carcinoma of the pleura, that is, the pleural effusion is primarily due to invasion of the lymphatic system. Moreover, the large bulk of tumor on the pleural surface would be expected to interfere with the removal of pleural fluid by the parietal pleural lymphatics, even if the lymphatics were not directly involved with tumor.
Benign asbestos pleural effusion probably develops as a result of the pleural inflammation that occurs during the passage of asbestos fibers across the pleural space to the parietal pleural lymphatics.
CLINICAL PRESENTATION
Patients with carcinoma involving the pleura most often present with symptoms attributable to a large pleural effusion—dyspnea on exertion and cough. The presence and degree of dyspnea depends on the size of the effusion and the patient’s underlying pulmonary function. A therapeutic thoracentesis results in relief of dyspnea in most patients.14 However, the volume of pleural fluid removed at thoracentesis does not correlate with the change in lung volume. The increase in total lung capacity (TLC) approximates one-third of the volume of fluid removed, while the forced vital capacity (FVC) increases to about one-half of the TLC. Indeed, the mechanism of dyspnea caused by a large pleural effusion appears to be multifactorial in origin, probably entailing a decrease in the compliance of the chest wall, a contralateral shift of the mediastinum, inversion of the ipsilateral diaphragm, and a decrease in ipsilateral lung volume modulated by neurogenic reflexes from the lungs and chest wall. Other factors that may contribute to dyspnea and cough are postobstructive pneumonia or atelectasis due to a central bronchial lesion, or the presence of infiltrative malignant disease of the pulmonary parenchyma.15
Since malignant involvement of the pleura signifies far advanced disease, these patients commonly have substantial weight loss and appear chronically ill. Chest pain may be present because of involvement of the parietal pleura, ribs, or chest wall. However, in a large series of patients with metastatic carcinoma of the pleura, almost 25% were “asymptomatic” at the time of presentation. In these patients, the malignant pleural effusion was first suspected on physical examination or diagnosed on routine chest radiograph. In almost 50% of patients, the pleural effusion was the first indication of cancer.
The respiratory symptoms of patients with pleural effusion due to lymphoma are indistinguishable in nature and frequency from those due to carcinoma. About 20% of patients with lymphoma have no respiratory symptoms when the malignant pleural effusion is diagnosed.
Most patients with carcinoma of the pleura have evidence of a pleural effusion on physical examination when first seen by the physician. Physical signs of pleural effusion are to be expected, since the volume of pleural fluid in most malignant effusions is greater than 500 mL. Cachexia and lymphadenopathy are present in about one-third of patients on initial presentation. Ipsilateral chest wall tenderness and a pleural friction rub are rare.
In contrast to patients with carcinomatous involvement of the pleura, virtually all patients with malignant mesotheliomas are symptomatic when first seen by the physician. In six series of patients encompassing 160 cases of malignant mesothelioma, only one patient was asymptomatic at presentation.10–12 Chest pain is the most common presenting symptom and occurs in 60% to 70% of patients. Dyspnea and cough are next in frequency and are present in about 25% and 20% of patients, respectively.
Benign pleural effusion due to asbestos exposure is a diagnosis of exclusion. Its frequency of occurrence in exposed workers is estimated to be up to 7%. Benign asbestos pleural effusion is the most common manifestation of asbestos-related pleuropulmonary disease in the first 20 years after initial asbestos exposure. Two-thirds of patients with benign asbestos pleural effusions are asymptomatic at presentation, with the effusion diagnosed on a routine chest radiograph. Approximately 20% of patients present with pleuritic pain and 10% with dyspnea.16 The effusion generally persists for several months and resolves within 1 year. Recurrent effusions, either on the ipsilateral or contralateral side, occur in approximately 25% of patients. The differential diagnosis centers around distinguishing benign asbestos pleural effusion from mesothelioma. Since benign asbestos pleural effusion occurs sooner after initial exposure than does mesothelioma (20 years being the rough dividing line), a pleural effusion in a young asbestos-exposed individual is more likely to represent a benign effusion than is an effusion that occurs 20 to 40 years after initial exposure.16 Also, an asymptomatic pleural effusion is more apt to be benign. The absence of other radiographic manifestations of asbestos exposure is not helpful in distinguishing between a benign effusion and mesothelioma. Preoccupation with asbestos-related disease occasionally leads to overlooking treatable causes of pleural effusion, such as tuberculosis.
CHEST RADIOGRAPHY
A pleural effusion ipsilateral to the primary lesion is the rule in carcinoma of the lung. When the primary site of the cancer is elsewhere than the lung and with the possible exception of breast cancer, there seems to be no ipsilateral predilection and bilateral effusions are common.
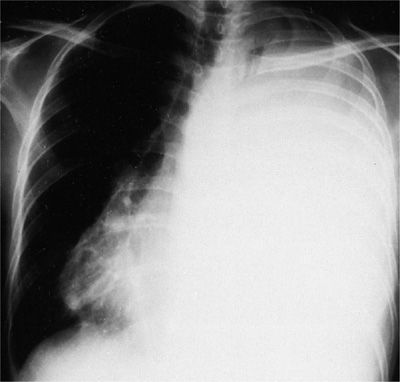
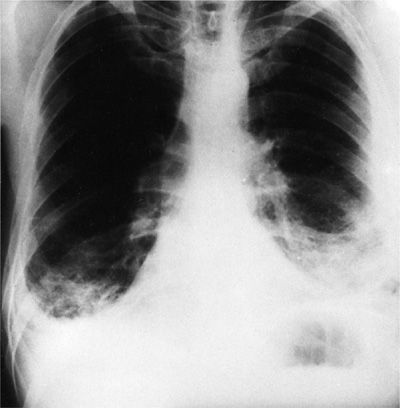
In three of four patients who present with carcinomatous involvement of the pleura, the pleural effusion is moderate to large, with volumes ranging from 500 to 2000 mL of fluid. Approximately 10% present with effusions of less than 500 mL; another 10% present with massive pleural effusions (complete opacification of the hemithorax) (Fig. 77-2). Some 70% of patients with a massive pleural effusion have a malignancy.
Figure 77-2 Carcinoma of the cervix metastatic to the left pleura and mediastinum. The massive pleural effusion is associated with a contralateral shift of the mediastinum.
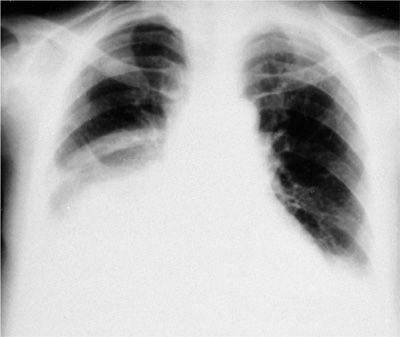
The finding of bilateral effusions with a normal heart size also suggests a malignant etiology (Fig. 77-3). Approximately 50% of patients who present with this radiographic finding have a malignant effusion. However, lupus pleuritis, hypoalbuminemia, constrictive pericarditis, rheumatoid pleurisy, benign asbestos pleural effusions, and cirrhosis must also be considered in the differential diagnosis.
Figure 77-3 Carcinoma of the lung involving right lower lobe, with metastasis to right pleura and mediastinal lymph nodes. The pleural effusions are bilateral and the heart size is normal.
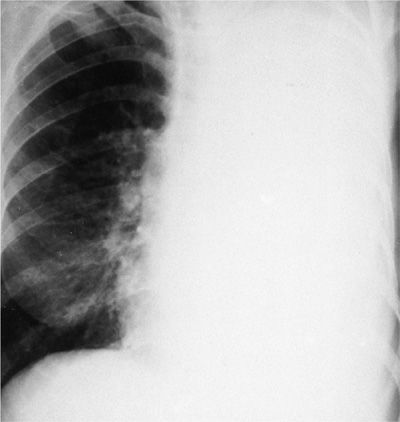
If the mediastinum does not shift contralaterally in the face of a large pleural effusion (>1500 mL), malignancy is highly likely. The following diagnoses are then considered: (1) Carcinoma of the ipsilateral mainstem bronchus resulting in atelectasis (Fig. 77-4); (2) a fixed mediastinum due to malignant lymph nodes; (3) malignant mesothelioma (the radiodensity represents predominantly tumor with only a small effusion); and (4) extensive tumor infiltration of the ipsilateral lung, radiographically mimicking a large effusion. Interstitial infiltrates with effusions (lymphangitic carcinomatosis) and multiple nodules with effusions also suggest malignant disease.
Figure 77-4 Carcinoma of the left mainstem bronchus resulting in complete atelectasis of the left lung. The left hemithorax is completely opacified, and the mediastinum has shifted to the side of the bronchial occlusion. The radiographic opacity represents a combination of collapsed lung and pleural fluid.
Depending on the stage of the mesothelioma at the time of presentation, the chest radiograph may show a moderate to large pleural effusion (early) or a nodular, thickened pleura with extension to the apex of the hemithorax (late). Contralateral mediastinal shift often occurs early, that is, when the pleural effusion is large; but, as fluid resorbs and is replaced by tumor, the ipsilateral hemithorax shrinks in size and the mediastinal structures either remain in the midline or shift ipsilaterally (Fig. 77-1).10–12 Contralateral manifestations of asbestos-induced pleuropulmonary disease, such as pleural plaques with or without calcification and interstitial lung disease, often reinforce the diagnosis. In the more advanced stages of malignant mesothelioma, other radiographic findings are mediastinal widening due to lymph node involvement, an enlarged cardiac silhouette due to pericardial involvement with effusion, and extrapleural lesions, such as soft tissue masses or rib destruction.
Benign asbestos pleural effusions are small to moderate (<1000 mL) unilateral effusions with evidence of pleural plaques or asbestosis identifiable in less than 20% of patients.16 Calcified pleural plaques are rare, since calcifications require 25 to 40 years from the time of initial asbestos exposure, whereas benign asbestos pleural effusion tends to be the earliest manifestation of asbestos pleuropulmonary disease. Some patients are left with normal chest radiographs, but most have residual abnormalities. These include a blunted costophrenic angle (most common), crow’s feet (converging fibrous strands creating a likeness of a bird’s foot), rounded atelectasis (in which a portion of the lung periphery has become atelectatic due to pleural adhesions that collapse small bronchi), and diffuse pleural thickening that is sometimes progressive (Fig. 77-5).
Figure 77-5 Bilateral pleural thickening in a 44-year-old man exposed to asbestos for 18 months 20 years ago. Bilateral pleural effusions were succeeded by progressive pleural thickening.
PLEURAL FLUID CHARACTERISTICS
Malignant pleural fluid may be serous, serosanguineous, or grossly bloody. The number of nucleated cells in the pleural fluid is modest (1500–4000/μL) and consists of lymphocytes, macrophages, and mesothelial cells. In about one-half of malignant pleural effusions, lymphocytes predominate (50% –70% of nucleated cells). Malignant cells in pleural fluid are rare in some patients; in others, they constitute virtually the complete population. Polymorphonuclear leukocytes usually represent less than 25% of the cell population; but, rarely, when pleural inflammation is active, they predominate. The reported prevalence of pleural eosinophilia in malignant effusions ranges from 8% to 12%.17 However, malignancy was as frequent in eosinophilic as noneosinophilic pleural effusions. Therefore, the finding of pleural fluid eosinophilia should not be considered a predictor of benign disease.
The pleural fluid in patients with carcinoma of the pleura is typically an exudate with a protein concentration of about 4 g/dL. However, protein concentrations have been reported in the range of 1.5 to 8.0 g/dL. Often unappreciated is the fact that less than 5% of malignant pleural effusions can be transudates. These transudates are due either to concomitant congestive heart failure, atelectasis from tumor obstructing a major bronchus, or the early stages of lymphatic obstruction. Since protein can exit from the pleural space only by parietal pleural lymphatics, a few weeks are necessary for protein to accumulate (from the 1.5 g/dL of normal pleural liquid) to a level of greater than 50% of the serum concentration. Chronic pleural effusions and those with a low pleural fluid pH and glucose tend to have a higher total protein concentration and are virtually never transudates. Sometimes, the total protein pleural fluid to serum ratio may be low (<0.50), but the fluid would qualify as an exudate by lactate dehydrogenase (LDH) criterion alone.
In about one-third of patients with malignant pleural effusions at the time of diagnosis, the pleural fluid pH is low (<7.30), ranging from 6.95 to 7.29. In these low-pH effusions, the glucose concentration is also low (<60 mg/dL, or the ratio of pleural fluid to serum glucose is below 0.5), the lactate concentration is high, the PCO2 is high, and the PO2 is low.1,18 As a rule, the glucose concentrations are in the range of 30 to 55 mg/dL though on rare occasions, the glucose is as low as 5 mg/dL. These low-pH, low-glucose effusions have usually been present for several months and are associated with a large tumor burden and fibrosis of the pleura. The markedly abnormal pleura interferes with glucose transport from blood to pleural fluid; the glucose that does enter is metabolized by normal and malignant pleural cells to form CO2 and lactate. The abnormal pleura impairs the efflux of these end products of glucose metabolism from the pleural space, resulting in pleural fluid acidosis. About 10% of malignant pleural effusions have high amylase concentrations. The finding of a high level of salivary-like isoamylase in a patient without esophageal rupture essentially establishes the diagnosis of malignancy, most likely adenocarcinoma of the lung.
Early in the course of malignant mesothelioma, the pleural fluid may be serous; later, it tends to be hemorrhagic. The effusion associated with malignant mesothelioma is an exudate with a protein concentration in the range of 4 to 5 g/dL and a modest number of nucleated cells (<5000/μL), predominantly mononuclear. The LDH concentration tends to be higher than in the patient with carcinoma of the pleura; frequently the concentration exceeds 600 IU/L. In 60% of patients with malignant mesothelioma, at the time that the diagnosis is established, the pleural fluid pH is low (below 7.30) and the glucose concentration is also low (pleural fluid/serum ratio below 0.5); in contrast, the incidence of low pH and low glucose concentration in carcinoma of the pleura is about 30%. The natural progression of malignant mesothelioma, resulting in large tumor masses and concomitant fibrosis that obliterate the pleural membrane, provides a reasonable explanation for these biochemical findings. In some instances of malignant mesothelioma, the viscosity of pleural fluid is greatly increased because of a high concentration of hyaluronic acid. Although a high concentration of hyaluronic acid in pleural fluid does raise the question of malignant mesothelioma as the cause, this test is not specific and only moderately sensitive; thus, it is of no diagnostic value.
The pleural fluid in benign asbestos pleural effusions is a sanguineous, lymphocyte-predominant exudate, with pleural fluid eosinophilia in 30% of cases. During the acute stage, there may be a moderate number of polymorphonuclear leukocytes. The pH and glucose are in the normal range (above 7.30 and 60 mg/dL, respectively).
DIAGNOSIS
A malignant pleural effusion can be diagnosed only by demonstrating malignant cells in pleural fluid or pleural tissue. Cytology is a more sensitive test for the diagnosis than percutaneous pleural biopsy, because pleural metastases tend to be focal and the latter is a blind sampling procedure. The yield of either procedure increases as the disease becomes more advanced. It appears, based on thoracoscopy, that initial pleural metastases originate near the mediastinum and diaphragm; as the disease progresses, tumor spreads cephalad and costally. The yield from pleural biopsy with a proven malignant effusion averages 50% to 60%. With improved techniques, the yield from exfoliative cytology now approaches 90% to 95%. If the clinician suspects a malignant effusion, several hundred milliliters of fluid should be removed at the initial diagnostic thoracentesis and at least 60 mL sent for cytological examination.19 This maneuver will not improve the yield on the initial study but, if it is negative, a repeat procedure several days later may provide fluid with fewer degenerative mesothelial cells and freshly exfoliated malignant cells. Percutaneous pleural biopsy should be reserved for the second thoracentesis if the initial pleural fluid cytological examination is negative. If the second cytological examination and initial pleural biopsy are negative, a third cytological examination and second pleural biopsy soon after, usually is not diagnostic.
There are several options for the patient with suspected malignancy and a negative pleural fluid and pleural tissue examination. These include observation for a few weeks with repeat studies, thoracoscopy, or open pleural biopsy. Before proceeding to more invasive procedures, other causes of an exudative pleural effusion must be excluded. Tuberculous pleurisy should always be considered in the patient with a lymphocyte-predominant exudate with or without a positive tuberculin skin test. The yield from pleural biopsy culture and histology, in conjunction with pleural fluid culture, should provide a bacteriological diagnosis of tuberculous pleurisy in 90% to 95% of cases. Even if diagnostic studies are negative, patients with a positive purified protein derivative skin test or interferon-γ release assay and a lymphocyte-predominant exudate should be treated presumptively for tuberculous pleurisy because of the high risk (43%–65%) of developing active pulmonary or extrapulmonary tuberculosis within 5 years if untreated. Bronchoscopy has a low diagnostic yield for an idiopathic pleural effusion without parenchymal lesions on chest radiograph, ipsilateral mediastinal shift, or hemoptysis. The value of computed tomographic examination of the chest in an undiagnosed exudative effusion is unknown and probably not cost-effective. If observation is the course undertaken, the clinician would expect a malignant pleural effusion to be stable or progress and an effusion not due to malignancy to be stable or regress over time. Failure to identify a malignant pleural effusion for several weeks is rarely a disservice to the patient, who has incurable disease. Exceptions are those malignancies that tend to be responsive to therapy, such as breast cancer, prostate cancer, thyroid cancer, small-cell lung carcinoma, germ-cell neoplasms, and lymphomas.
The diagnostic utility of immunohistochemistry in the diagnosis of malignant pleural effusions secondary to adenocarcinoma, mesothelioma, and lymphoma has been established. Carcinoembryonic antigen (CEA), Leu-M1, B 72.3, Ber-EP4, and BG-8 are the best markers for the diagnosis of adenocarcinoma.20 Calretinin and cytokeratin 5/6 are the best markers for mesothelioma.11,20 Flow cytometry is useful in the evaluation of lymphocytic pleural effusions in which lymphoma is a possible diagnosis.21 The ability of tumor markers to discriminate between benign and malignant pleural effusions is poor. Markers such as CEA, vascular endothelial growth factor (VEGF), carbohydrate antigens (e.g., CA 15-3, 19-9, and 72.4), cytokeratin 19, and enolase have significant overlap between benign and malignant pleural effusions. Hyaluronan does not appear to discriminate between pleural effusions from adenocarcinoma and mesothelioma.22
Inflammatory processes involving the pleura may mimic mesothelioma, and patients are often subjected to a battery of tests and consultations before the diagnosis is established. An accurate diagnosis is imperative for proper epidemiological records, appropriate therapeutic intervention, and litigation. Early in the course of a mesothelioma, establishing a definitive diagnosis may be problematic. Pleural fluid cytology and pleural biopsy may allow the diagnosis of malignancy but often cannot distinguish between mesothelioma and adenocarcinoma. Sarcomatous type mesothelioma can be confused with rare tumors, such as fibrosarcomas or hemangiopericytomas. Thoracoscopic biopsy or open thoracotomy is usually necessary to obtain adequate tissue to confirm the diagnosis. Thoracoscopic biopsy has a high diagnostic yield for mesothelioma, approaching 100% in some series, while the yield for pleural fluid cytology alone is 25% and that for combined pleural fluid cytology and closed pleural biopsy is 40%. Histochemical and immunochemical studies, in conjunction with electron microscopy, have improved the accuracy of the diagnosis of malignant mesothelioma.
PROGNOSIS
The diagnosis of a malignant pleural effusion signals a poor prognosis. Patients with carcinoma of the lung, stomach, and ovary tend to have a survival time of only a few months from the time that the malignant effusion is diagnosed; patients with breast cancer tend to survive longer, several months to years, depending on the response to chemotherapy. Patients with lymphomatous pleural effusions tend to have survival times intermediate between those of breast cancer and other carcinomas.
Several studies have suggested that when pH and glucose concentrations in the malignant pleural effusion are low (below 7.30 and 60 mg/dL, respectively), the survival time is less (average 2 months) than in those with a normal pH and glucose (average 10 months). However, in a systematic review of 433 patients with malignant effusions, pleural fluid pH was of marginal clinical utility in predicting survival less than 3 months.1 Performance status at the time of diagnosis may be the only true predictive marker for mortality. A Karnofsky score less than 30 is associated with a median survival of 1.1 months compared to a median survival of 13.2 months in association with a Karnofsky score more than 70.
A pleural effusion in the setting of lung cancer usually excludes operability; however, approximately 5% of these patients have a paramalignant effusion or an effusion from another cause and may be operable and curable. Thus, it is essential to establish the cause of the pleural effusion before deciding that the patient is not a candidate for curative surgery.
Survival following the diagnosis of malignant mesothelioma is related to the stage of the disease at the time of presentation. Those patients with only ipsilateral involvement of the pleura and lung survive the longest, whereas those with distant hematogenous metastases have the shortest survival. Chest pain portends a worse prognosis than dyspnea, reflecting a more advanced stage of disease. Overall, the median survival in malignant mesothelioma is about 9 months. The epithelial type has a median survival approximately twice that of the sarcomatous type; long-term survivors of more than 3 years are seen almost exclusively with the epithelial type. As in metastatic carcinoma of the pleura, a low-pH effusion in malignant mesothelioma is also predictive of a short survival with one study reporting an association with increasing pH and survival.
Benign asbestos pleural effusions tend to resolve within 3 to 4 months, leaving some residual on the chest radiograph. Although malignant mesothelioma occasionally develops in patients with benign asbestos effusions, it does not appear to be a harbinger of mesothelioma. Obviously, the risk of developing mesothelioma is greater in these asbestos-exposed individuals than in the general population.
TREATMENT
When the pleural effusion has been proved to be malignant or paramalignant and the patient is not a surgical candidate, the type of palliative therapy is weighed, taking into account the patient’s general condition, symptoms, performance status, primary tumor type and expected response to therapy, expected survival, and degree of lung reexpansion following pleural fluid evacuation. Several management options are available (Table 77-3 and Fig. 77-6). Asymptomatic patients need not be treated; however, most will develop progressive pleural effusions that will evoke symptoms and require therapy, but some will reach a steady state of pleural fluid formation and removal and not progress to a symptomatic stage. In the debilitated patient in whom a short survival is expected based upon the general health, extent of disease, and biochemical characteristics of the pleural fluid, periodic therapeutic thoracentesis as an outpatient is often preferable to hospitalization for tube thoracostomy and intrapleural instillation of a chemical agent. However, outpatient pleurodesis using small-bore catheters can be accomplished successfully with decreased cost and morbidity.