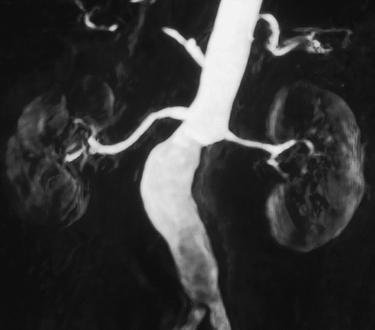
Magnetic resonance angiography (MRA) accurately assesses the caliber of the entire renal artery in multiple projections without subjecting the patient to risks of arterial catheterization, ionizing radiation, or nephrotoxic contrast (Figures 1 and 2). Gadolinium-based contrast agents are used for the highest-quality images with least artifact. These agents are free of nephrotoxicity at the doses used for the highest-quality images with least artifact in MRA and have a very low incidence of allergic reactions (1:20,000). For patients with severe renal insufficiency—glomerular filtration rate (GFR) less than 30 mL/min—several gadolinium-based contrast agents are contraindicated owing to a risk of causing nephrogenic systemic fibrosis.
During magnetic resonance imaging (MRI), the patient is placed within a strong magnetic field. A small fraction of nuclear spins within the patient’s atoms orient with this magnetic field. These spins oriented to the magnetic field can be manipulated with radiofrequency pulses to give off signal. This signal can be characterized by two time constants: T1 relaxation and T2 relaxation. Gadolinium contrast agents primarily affect T1 relaxation.
By shortening the T1 relaxation time of blood, gadolinium contrast agents make the blood bright. Capturing the arterial phase of a gadolinium contrast bolus injection on these T1-weighted images produces an arteriogram that is analogous to conventional arteriography. The highest relaxivity agent, gadofosveset trisodium, produces the greatest contrast effect and highest signal-to-noise ratio. It also has a blood pool distribution for excellent venous visualization.
MRA has an advantage of being inherently three-dimensional (3-D). The 3-D volume of MRI data acquired with a single contrast injection can be reformatted into any obliquity on a computer workstation, even after the patient has exited the magnet. This is an advantage because it becomes possible to obtain perpendicular and axial views, which might not even be possible with conventional catheter arteriography. This becomes especially useful in evaluating ectatic and tortuous or overlapping arteries.
The contrast effect on the image is formed primarily by the data in the center of the Fourier data array, also known as the center of k-space. MRA data acquisition strategies using radial or spiral k-space trajectories can oversample the center of k-space, making it easy to produce time-resolved movies of the gadolinium flowing down the aorta and out the renal arteries and eventually enhancing the renal veins. High-resolution angiograms at four frames per second have been demonstrated, and these have the ability to look at the differential flow and enhancement between right and left kidneys. Slower renal artery filling and parenchyma enhancement raises a suspicion for a significant ipsilateral renal artery stenosis.
Reasonable visualization of the renal arteries can also be obtained without contrast using the steady-state free-precession technique. This method is inherently flow compensated, which helps preserve the bright blood signal. Various strategies can be employed to eliminate competing bright signals from fat and venous blood to produce an arteriogram. Synchronization with respiration can eliminate respiratory motion in many patients who cannot hold their breath.
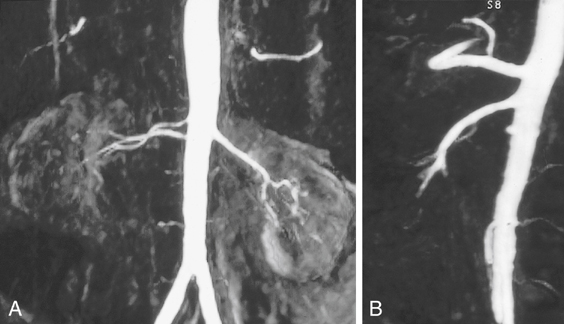
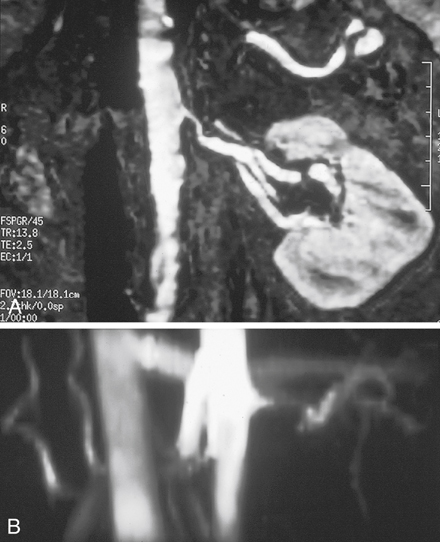
Renal MRI provides considerable information in addition to the arteriogram. Cross-sectional images can document kidney length and parenchymal thickness, as well as other pathological conditions in the abdomen that may be important for surgical planning. The 3-D phase contrast images document sites of signal dropout (dephasing) from the swirling jet flow that is associated with blood pressure gradients at stenoses (Figures 3 and 4). Signal dephasing on 3-D phase contrast at a stenosis and just distal to the stenosis indicates that the stenosis is hemodynamically significant.
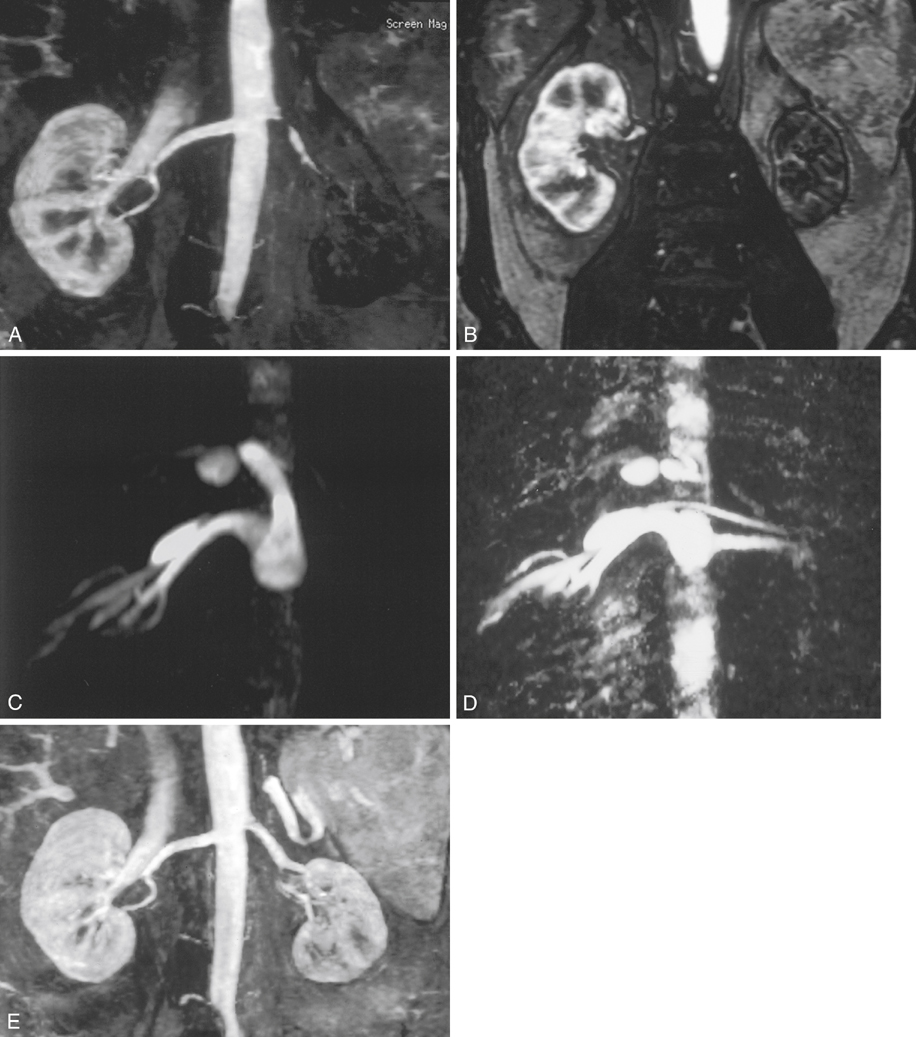
FIGURE 4 A, Coronal subvolume intensity projection of three-dimensional (3-D) gadolinium magnetic resonance angiography shows severe left renal artery stenosis and delayed arterial phase enhancement of left kidney before angioplasty. B, Coronal single slice during arterial phase also demonstrates the small size and minimal enhancement of the left kidney before angioplasty. C, Axial 3-D phase contrast shows no flow signal (dephasing) in the left renal artery before angioplasty. D, Axial 3-D phase contrast shows restored flow after angioplasty. E, After angioplasty there is restored arterial phase enhancement. (Reprinted with permission from Prince MR, Narasimham DL, Stanley JC, et al: Breath-hold gadolinium-enhanced MR angiography of the abdominal aorta and its major branches, Radiology 197:785–792, 1995.)
Only gold members can continue reading.
Log In or
Register to continue
Related

Stay updated, free articles. Join our Telegram channel