Introduction
Elevated serum cholesterol was the single most important cause of the 20th-century pandemic of ischemic heart disease, with the high-fat diet typical of many Western countries over the greater part of the 20th century extending more recently to non-Western countries. Modern cholesterol-lowering drugs (statins) can reduce risk more than any other single intervention.
Serum total and low-density lipoprotein cholesterol
Typical values of serum total and low-density lipoprotein (LDL) cholesterol in Western countries are high in comparison with those in agricultural and hunter-gatherer communities, because of the high saturated fat content of the Western diet. Average serum cholesterol concentrations (in men aged 45–60) are about 3–3.5mmol/L in hunter-gatherer societies and rural China (where heart disease is rare), but over much of the 20th century they were about 5.0 mmol/L in Japan, 5.5 mmol/L in Mediterranean populations, and 6 mmol/L in the USA, Britain and Northern Europe.1 Use of the term “normal” in reference to usual or average Western cholesterol values may therefore be misleading (we are all “high”). In recent years cholesterol levels in Japan have risen while those in the USA, Britain and Northern Europe have fallen a little. Average levels of LDL cholesterol are about 2 mmol/L lower than total cholesterol.1
Of the average total serum cholesterol in Western populations, two-thirds is LDL cholesterol and one-quarter is high-density lipoprotein (HDL) cholesterol. The atherogenic properties lie in the LDL fraction (sometimes measured as its carrier protein, apolipoprotein B, with which it is highly correlated). Many of the large epidemiologic studies and the earlier randomized trials measured only total serum cholesterol; total serum cholesterol has been used as a surrogate for LDL cholesterol. Fortuitously, the approximation is a good one. The absolute reduction in total serum cholesterol produced by diet and by most drugs (including statins2–12) is similar to the reduction in LDL cholesterol. Observational differences in total cholesterol between individuals in cohort studies are a little greater than the corresponding differences in LDL cholesterol, but this “surrogate dilution bias” can readily be corrected.13 Much epidemiologic and clinical trial data are therefore available to estimate quantitatively the effect of lowering serum LDL cholesterol on the risk of ischemic heart disease.
Serum cholesterol and ischemic heart disease
For about two decades we have had evidence from genetics, animal studies, experimental pathology, epidemiologic studies and clinical trials indicating conclusively that elevated serum cholesterol is an important cause of ischemic heart disease and that lowering serum cholesterol reduces the risk.14 Ten large randomized trials of statins, published over a 10-year period, have ensured that this is now widely accepted.2–12 Three important practical questions arise: the nature of the dose–response relationship, the size of the effect, and the speed of the reversal of risk. To answer these questions, data from both cohort studies (or prospective observational studies) and randomized controlled trials are necessary: the two are complementary. Table 11.1 summarizes the advantages of each.
Table 11.1 Relative advantages of cohort studies and randomized trials in assessing the relation between serum cholesterol and ischemic heart disease
| Objective | Advantage (comment) |
| Statistical power | Cohort studies (recorded seven times more ischemic heart disease events than the trials: 91 800 v 12 50015,16) |
| Dose–response relationship | Cohort studies (observation across a wide range of cholesterol values, from 3 to 14 mmol/L) |
| Wide age range | Cohort studies (ischemic heart disease events at ages 35–89, but mostly 55–65 in trials) |
| Long-term effects of cholesterol differences | Cohort studies (the cholesterol differences between individuals observed at the start of the study will have been present for decades previously) |
| Short-term effects of cholesterol differences | Randomized trials (on recruitment serum cholesterol was the same in intervention and control groups) |
| Avoid bias | Randomized trials (not a major advantage – bias in cohort studies can be allowed for) |
The nature of the dose–response relationship: is there a threshold?
A plot of ischemic heart disease mortality on total serum cholesterol in the Prospective Studies Collaboration (PSC) meta-analysis of 61 cohort studies showed a near-perfect straight-line dose–response relationship linking proportional differences in ischemic heart disease mortality (the vertical axis uses a logarithmic scale) to absolute differences in serum cholesterol, across the range of cholesterol values in Western populations15 (Fig. 11.1). Based on a single (baseline) cholesterol measurement, there was a continuous straight-line relationship between baseline total cholesterol values of 3 mmol/L and 14 mmol/L, using the baseline cholesterol measurements to divide the cohort into subgroups. However, using a repeat measure to estimate the mean cholesterol of each subgroup (“usual total cholesterol”), thereby allowing for regression dilution bias, the straight-line relationship extended between usual cholesterol values of 4 and 9 mmol/L. The straight line establishes that there is no threshold below which a further decrease in serum cholesterol is not associated with a further decrease in risk of ischemic heart disease. The exponential relationship indicated by the straight line means that a given absolute difference in serum cholesterol concentration from any point on the cholesterol distribution is associated with a constant proportional difference in the incidence of ischemic heart disease.
Figure 11.1 IHD mortality (33853 deaths) in categories defined only by the baseline cholesterol measurement versus: (a) risk versus a single measurement of cholesterol – mean of baseline values in each group and (b) risk versus resurvey (usual) cholesterol – mean of first resurvey values in each group (approximating mean usual values). Reproduced with permission from Prospective Studies Collaboration.15
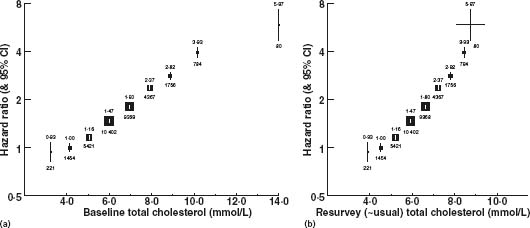
The absence of a threshold has in the past been contentious. However, the cohort study evidence (itself conclusive)15,16 is supported by subgroup analyses according to baseline cholesterol in a meta-analysis of randomized controlled trials showing similar proportional reduction in coronary heart disease (CHD) from all baseline levels,17 by randomized trials showing a greater CHD risk reduction and a greater degree of coronary artery atheroma regression with more intensive than less intensive LDL cholesterol reduction (from 2.6 to 2.0 mmol/L),18,19 by ecologic studies,1,14 and by experimental data on the transfer of cholesterol from the blood into atheromatous lesions which exclude a threshold as low as 1 mmol/L.20 People at higher risk of an ischemic heart disease event should be offered a statin irrespective of their existing level of total or LDL cholesterol.
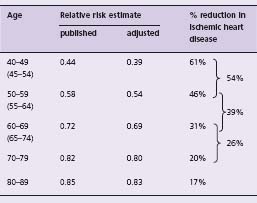
Table 11.2 shows estimates of the long-term percentage decrease in the risk of an ischemic heart disease event according to age at event, from the Prospective Studies Collaboration. A reduction in total or LDL cholesterol of 1 mmol/L is associated with a decrease in risk of ischemic heart disease of about 61% at age 45, 46% at 55, 31% at 65, 20% at 75 and 17% at 85. Statins (for example, simvastatin 40 mg, atorvastatin 10 mg) reduce LDL cholesterol by 1.8mmol/L;16 from the dose–response relationship this would reduce the risk of ischemic heart disease events by about 60% on average in people aged 50–69 years.
Table 11.2 PSC age-specific estimates of relative risk of ischemic heart disease for a 1 mmol/L lower total serum cholesterol,15 and estimates adjusted for the surrogate dilution bias,13 both for a 1 mmol/L lower LDL cholesterol
Speed of reversal and consistency of observational and trial data
Efficacy of serum cholesterol reduction according to duration has been determined from the results of randomized trials by separate meta-analysis of all CHD events occurring in the first year of trials, all occurring in the second year of trials, etc.16 Results are summarized in Table 11.2, applicable to age 60 (the average age of sustaining an isch-emic heart disease event in the trials). The estimate of the long-term effect of cholesterol reduction, from the cohort studies (in which the cholesterol differences between individuals are longstanding), is also shown. The proportional reduction in risk increases continuously with the duration of the cholesterol reduction: there is relatively little effect (11% reduction) in the first year, but a 33% reduction by the third year and (from cohort studies) a 39% reduction in the long term. The similarity of the estimates of effect from the cohort studies and from the trial data from the third year onwards indicates that the reversal of risk is near maximal after two years – a surprisingly rapid effect.
The estimates of effect are similar in men and women, in people with and without previous ischemic heart disease, and for coronary death and non-fatal myocardial infarction.15,17 Four randomized controlled trials have attained average LDL cholesterol reductions (treated minus placebo) exceeding 1.5 mmol/L (1.6mmol/L on average).16 In these four trials the average reduction in incidence of ischemic heart disease events from the third year onwards was 51% (95% confidence interval (CI) 42–58%, compared to an expected value from cohort studies of 55%).16 Trial data therefore show directly that LDL cholesterol reductions attainable with statins reduce risk by over half. Importantly, most randomized trials of statins do not show their full potential for preventing ischemic heart disease events because of “contamination” – some patients allocated to the treated group leave the trial and stop taking the tablets, some patients allocated to the placebo group take statins, and the results are necessarily analyzed on an intention-to-treat basis. The randomized trials therefore confirm the dose–response relationship shown in the cohort studies (the greater the cholesterol reduction, the greater the reduction in heart disease events16) and confirm the estimates from the cohort studies in Table 11.2 of the long-term reduction in risk.
As a parallel to the observation that a few years are necessary before serum cholesterol reduction is fully effective, follow-up of the participants in one randomized trial after the trial had terminated showed that the protective effect persisted in previously treated patients for some years after the termination of the trial (LDL cholesterol was similar in the former treated and placebo groups during this follow-up period).21
Dietary fat and serum cholesterol
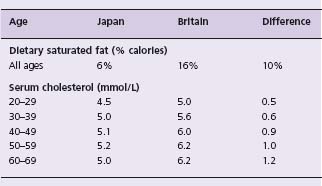
The relationship between dietary saturated fat and serum cholesterol is shown by the data from Japan and Britain in Table 11.3. This comparison is a useful one because dietary saturated fat differs greatly, yet dietary polyunsaturated fat and cholesterol are similar in the two countries, so the serum cholesterol difference is attributable to the saturated fat difference. As in other situations (salt and blood pressure, for example), the size of the association varies with age, yet there has been a tendency to generalize to older age groups the results of studies conducted in younger age groups. Many dietary trials, for example, have been conducted in people under 30. The few that have been conducted in people over 50 tend to support the above Japan–Britain comparison.14 In older people a reduction in dietary saturated fat equivalent to 10% of calories will lower serum cholesterol by about 1 mmol/L, which in turn will reduce ischemic heart disease mortality in the long term by about 40%.
Table 11.3 Decrease in ischemic heart disease events for a cholesterol decrease of 1 mmol/L, according to duration of the cholesterol decrease, from randomized trials16 and cohort studies15
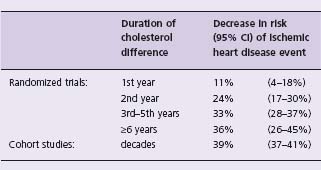
Table 11.4 Serum cholesterol and dietary saturated fat in J apan and B ritain. Data compiled from national surveys in each country1
The chain lengths of saturated fatty acids influence the extent to which they increase blood cholesterol. Palmitic (C 16:0) and myristic (C 14:0) acids have the major effect, lauric acid (C 12:0) some effect, and stearic acid (C 18:0) and medium chain fatty acids have little or no effect.
Trans unsaturated fatty acids are also important: randomized trials show that they increase serum total and LDL cholesterol by about as much as these longer chain saturated fatty acids.22,23 They are scanty in naturally occurring fats but are generated by the hydrogenation of vegetable oils for use as hardening agents in manufactured foods. They constitute 6 – 8% of dietary fat, or 2% of calories, in Western diets.
Naturally occurring cis unsaturated fatty acids reduce serum cholesterol by approximately half as much as longer chain saturated fatty acids increase it. Reduction in dietary cholesterol has a small effect on blood cholesterol concentration. Substitution of cis unsaturated for saturated fats in the Western diet is thus the most effective change in lowering the high levels of blood cholesterol in Western populations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


