Left Ventricular Outflow Tract Obstruction and Valvar Aortic Stenosis
Tara Karamlou
Gordon A. Cohen
VALVAR AORTIC STENOSIS
Pathology
Valvar aortic stenosis in children is most commonly congenital in etiology. The leaflets are thickened and dysplastic, with variable degrees of commissural fusion. The valve is typically bicuspid in morphology but may be tricuspid or even unicuspid. Stenosis of the right and left cusps is associated most frequently with fusion. Obstruction results from decreased leaflet mobility and a reduction in effective orifice size. Small annular size may also be present, further impeding left ventricular ejection.
Clinical Presentation and Diagnosis
Neonates with critical aortic stenosis develop symptoms of congestive heart failure and impaired systemic perfusion consequent to closure of the ductus arteriosus. Prompt diagnosis and institution of therapy are, therefore, necessary to prevent rapid deterioration and death.
Older children present less acutely, often with the finding of an asymptomatic heart murmur on routine physical examination. Feeding difficulty in infants and decreased exercise tolerance in older children may be observed as the severity of obstruction increases. Later symptoms include exertional angina, congestive heart failure, and syncope. Physical findings generally are limited to the cardiovascular examination: a harsh systolic ejection murmur at the right upper sternal border radiating to the neck, S4 gallop, and poor upstroke of the carotid pulse. An ejection click may indicate the presence of a bicuspid valve.
Chest X-ray is usually nondiagnostic. The electrocardiogram may show left ventricular hypertrophy. Echocardiography is the principal diagnostic method for the evaluation of left ventricular outflow tract obstruction (LVOTO). Two-dimensional echocardiogram allows assessment of the LVOT and aortic valve morphology including leaflet number, degree of thickening, and mobility. In addition, left ventricular cavity size, the presence and severity of left ventricular hypertrophy, and ventricular function can be assessed. Color Doppler imaging accurately identifies the level of obstruction, allowing a distinction between valvar, subvalvar, and supravalvar stenosis. Doppler measurement of blood flow velocity across the valve provides an estimate of the peak pressure gradient. Progression of disease and timing of intervention, therefore, can be determined in a noninvasive manner in most cases.
With accurate echocardiographic assessment, cardiac catheterization is rarely required in the diagnostic evaluation of aortic stenosis. Direct simultaneous measurement of left ventricular pressure and aortic pressure is the most accurate method for assessing the outflow tract gradient. However, identification of the precise level of obstruction may not be possible. Elevation of left ventricular end-diastolic pressure indicates impaired diastolic function. Left ventriculography allows assessment of ventricular systolic function and may outline the valve leaflets, providing some assessment of morphology.
Treatment
Neonatal Critical Aortic Stenosis
Critical aortic stenosis diagnosed in the newborn period constitutes a medical emergency. A neonatal presentation indicates severe outflow obstruction that requires urgent intervention. Initial stabilization includes endotracheal intubation and inotropic support. Prostaglandin infusion will establish or maintain patency of the ductus arteriosus and improve systemic perfusion. Emergency aortic valvotomy previously was the treatment of choice, performed soon after resuscitation. In the current era, percutaneous transcatheter balloon aortic valvotomy has supplanted surgical valvotomy in most centers. Transcatheter valvotomy is performed in the catheterization laboratory, with the advantages of rapid relief of obstruction and avoidance of cardiopulmonary bypass and aortic cross-clamping. Regardless of the technique, neonatal aortic valvotomy is considered a palliative procedure for most patients, with 41% undergoing reintervention within 10 years.
In a small subset of neonates with critical aortic stenosis and small left ventricular size, it may be difficult to determine whether a biventricular approach with aortic valvotomy/replacement or a single-ventricle approach with the Norwood procedure is more appropriate. Several studies have attempted to identify preoperative predictors for the suitability of single ventricle versus biventricular repair in this challenging population. However, none of these studies have established a universally adopted set of criteria, and patient triage to a single versus biventricular pathway is the subject of a current multi-institutional study sponsored by the Congenital Heart Surgeons Society.
Neonates with Multilevel Left Ventricular Outflow Tract Obstruction
Neonates with multiple levels of LVOTO are a complex group. Surgical options must be tailored to the particular anatomic considerations (Fig. 84.1). Neonates undergoing single-ventricle palliation can be managed with a staged approach, consisting of a Norwood or modified Norwood operation. Hybrid palliation, with implantation of a ductal stent and concomitant bilateral pulmonary arterial banding, is another recently developed option. This strategy, however, is currently reserved for marginal candidates with contraindications to standard Norwood-type palliation, or as a bridge to heart transplantation, with few centers using Hybrid palliation as the preferred approach. Heart transplantation is an attractive strategy for infants with severe atrioventricular valve regurgitation,
visceral heterotaxy, or poor systemic ventricular function.
visceral heterotaxy, or poor systemic ventricular function.
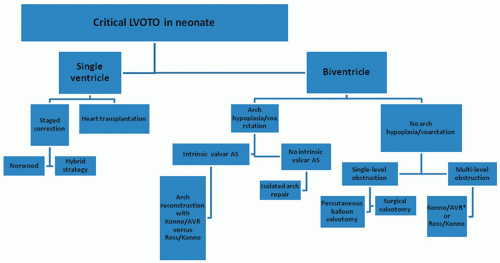 Fig. 84.1. Treatment algorithm for neonates and infants with critical left ventricular outflow tract obstruction. |
Neonates undergoing biventricular repair have several potential options depending upon the associated intracardiac and arch pathology. Infants with combined valvar aortic stenosis and aortic arch obstruction can be managed with standard patch aortoplasty concomitant with either a neonatal Ross operation or a mechanical aortic valve replacement with an annular enlargement procedure (Konno aortoventriculoplasty). Our institutional preference has been to manage these patients with aortic valve replacement and Konno, as results with the neonatal Ross operation have been poor, especially in neonates with associated mitral valve abnormalities. The presence of a ventricular septal defect adds an additional level of complexity, with options including those discussed above, arch repair with closure of the ventricular septal defect, or Yasui reconstruction.
Valvar Aortic Stenosis in Older Children
The objectives of surgical treatment of aortic stenosis are relief of symptoms and reduction of the risk of sudden death. Sudden death is related directly to the severity of obstruction and correlates with the peak systolic gradient, although it is reported to be <1%. Stenosis is considered severe when the mean echocardiographic gradient is ≥40 mmHg, moderate when it is between 25 and 40 mmHg, and mild when it is <25 mmHg. Surgery is indicated in any symptomatic patient regardless of severity and in asymptomatic patients with severe stenosis. Although surgery is not recommended for asymptomatic patients with mild stenosis, there is controversy about the management of asymptomatic patients with moderate stenosis. Surgical management should be considered on an individual basis. The authors generally recommend surgery in patients who demonstrate reduced left ventricular function, a strain pattern on electrocardiography, or abnormal exercise testing.
The two options for surgical management of aortic stenosis are valvotomy and aortic valve replacement. Valve replacement in children poses unique challenges that are not seen in the adult population. The smallest mechanical and bioprosthetic valves available (15 to 19 mm) may be too large for the annular size of smaller children. Even when replacement is feasible, unless an “adult-sized” valve is implanted, somatic growth eventually results in recurrent outflow obstruction due to patient-prosthesis mismatch. In addition, re-replacement with an appropriately sized valve may be limited by restricted annular growth from the original prosthesis. These limitations may necessitate concomitant performance of an annulus-enlarging procedure such as a Konno aortoventriculoplasty. Posterior enlargement procedures such as the Nicks or Manougian procedures are often insufficient in children. These options are discussed in detail in later sections.
The choices of prosthetic valves for children are more limited than are those for adults. Bioprostheses do not require anticoagulation, but calcific degeneration occurs at an accelerated rate compared with adults, limiting their use in children. Mechanical prostheses maintain their structural integrity and generally have excellent freedom from reintervention or re-replacement, but require anticoagulation with warfarin. Anticoagulation can be achieved safely in children, but issues of compliance, inconsistent diet, and potential for injury increase the risk of bleeding and thromboembolic complications in this patient population. A recent paper by Alsoufi and colleagues demonstrated that the use of either homograft or bioprosthetic valves in children was associated with an increased risk of valve-related reoperation (82% at 15 years) compared with both mechanical valve implantation and the Ross operation. However, long-term survival was excellent (86% at 15 years) in patients having homograft or bioprosthetic valve replacement, underscoring that these valve types can be useful in female patients or those in whom anticoagulation cannot be administered.
Allograft replacement of the aortic root became popular in the early 1990s. Small valves with excellent early hemodynamic performance were readily available, and it was hoped that degeneration would not occur. Subsequent reports, however, demonstrated severe accelerated degeneration that required early reoperation in younger children. Severe calcification and inflammatory reaction of surrounding tissues complicate allograft removal and place adjacent structures, including the mitral valve and the bundle of His, at risk during reoperation.
Pulmonary autografting and reconstruction of the right ventricular outflow tract with an allograft (the Ross procedure) are often the best choice for aortic valve replacement in small children. The autograft has growth potential, does not develop calcific degeneration, and does not require anticoagulation. There are, however, a number of important limitations. The aortic and pulmonary annulus sizes must be similar; stretching of the autograft to fit a dilated aortic root will result in neoaortic insufficiency. The presence of a bicuspid aortic valve may increase the propensity for autograft failure given the associated aortopathies in this population. The operation is technically challenging, requiring longer periods of cardiopulmonary bypass and aortic cross-clamping compared with other techniques for valve replacement. Allograft reconstruction of the right ventricular outflow tract necessitates eventual reintervention (either surgical or transcatheter) to relieve conduit stenosis from somatic growth and calcific degeneration. Finally, it has been recognized that the pulmonary autograft develops important dilation over time out of proportion to somatic growth, and that this dilation eventuates in progressive neoaortic insufficiency.
In summary, there is no ideal substitute for aortic valve replacement in children with valvar aortic stenosis. Aortic valvotomy is, whenever feasible, the procedure of choice in this population. Although often not definitive, adequate palliation is usually achieved. Small residual gradients and mild aortic insufficiency are acceptable. Valvotomy can defer the need for valve replacement significantly, allowing insertion of a larger prosthesis at a later time and reducing the total number of re-replacements required over a patient’s lifetime.
Surgical Procedures
Aortic Valvotomy
Surgical aortic valvotomy is usually performed via a median sternotomy. A single right atrial cannula provides venous drainage, and the aortic cannula is placed as distally as possible. Some surgeons advocate closed valvotomy that is performed by passing dilators of increasing size (usually up to 1 mm larger than the preoperative echocardiographic aortic annulus diameter) through the aortic valve; the dilators are introduced through a purse string in the left ventricular apex. This technique can be performed through a left thoracotomy without the need for cardiopulmonary bypass. The authors believe that open valvotomy with direct visualization of the aortic valve allows more precise leaflet separation, resulting in better relief of gradient and a decreased risk of significant aortic insufficiency.
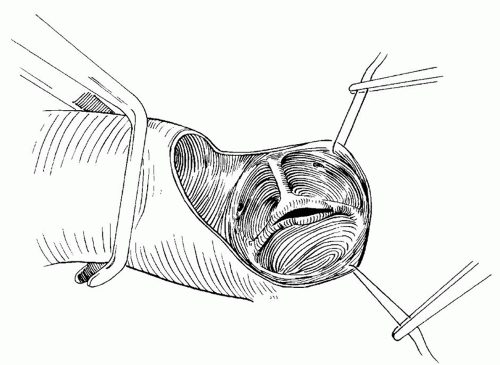
The aorta is clamped, and cardioplegia is delivered via the aortic root. A transverse aortotomy is made just above the sinotubular junction, with care taken to avoid injury to the right coronary artery and the aortic valve leaflets. The valve is inspected carefully. Fused commissures are opened precisely with a scalpel (Fig. 84.2). The incisions are not extended to the aortic wall, since this may result in loss of support and aortic insufficiency (Fig. 84.3). Excessive fibrous tissue, if present, is excised from the leaflets. Fibrous attachments between the base of the leaflets and the aortic wall are divided to maximize leaflet mobility. Despite these steps, the effective orifice may be inadequate, particularly in smaller bicuspid valves. Ilbawi and colleagues described a technique of extended valvotomy for these patients. Circumferential incisions are made above the true commissures and raphes. They reported a low incidence of aortic insufficiency and significantly lower gradients compared with standard valvotomy. The aortotomy is closed precisely to avoid supravalvar narrowing, and the cross-clamp is removed. The patient is weaned from cardiopulmonary bypass with mild inotropic support (dopamine 5 µg/kg/min and milrinone 0.5 µg/kg/min). The adequacy of repair is assessed by transesophageal echocardiography and direct measurement of pressure in the left ventricle and ascending aorta. Modified ultrafiltration is performed before decannulation.
Early mortality in older infants and children undergoing aortic valvotomy is reported currently to be <2%, and late mortality is rare. Reintervention for progressive regurgitation or restenosis may be required, but this generally occurs later than in neonates. The reoperation rate in children over 1 year of age at the time of the initial valvotomy is 2% at 10 years but then increases 3.3% per year.
Aortic Valve Replacement
Aortic valve replacement is required when valvotomy is not sufficient to reduce the transvalvar gradient adequately, or in patients with greater than mild aortic insufficiency. In larger children with adequate annular size, simple replacement of the valve is performed as it is in adults. Prosthesis selection should be individualized to the patient’s lifestyle and activity level. The authors generally avoid porcine and bovine bioprostheses as well as
allograft implantation because of the rapid degeneration and early failure observed in children. The remaining options, therefore, are limited to mechanical prosthesis and pulmonary autograft.
allograft implantation because of the rapid degeneration and early failure observed in children. The remaining options, therefore, are limited to mechanical prosthesis and pulmonary autograft.
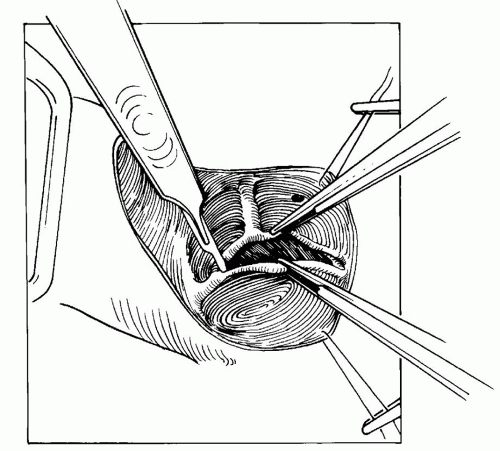 Fig. 84.3. A valvotomy is performed by incising the fused commissures. Care is taken to avoid incisions in false raphes, which may result in valve insufficiency. |
Aortic valve replacement with a mechanical prosthesis is performed via a median sternotomy. A single right atrial or two-stage venous cannula provides venous drainage, and the ascending aorta is cannulated distally. The aorta is clamped, and in the absence of significant aortic insufficiency, cardioplegia is infused in the aortic root. After aortotomy, additional cardioplegia is delivered every 30 minutes through a retrograde coronary sinus catheter or by direct coronary ostial perfusion. A left ventricular vent is placed through the left atrial appendage or the right superior pulmonary vein.
A transverse aortotomy is made and is extended into the noncoronary sinus of Valsalva. The aortic valve leaflets are excised. Interrupted pledgeted mattress sutures are placed circumferentially around the annulus. In smaller patients, intra-annular placement may be preferable to avoid coronary ostial obstruction by the sewing ring. The sutures are passed through the sewing ring, and the valve is parachuted into place. After the sutures are tied, the prosthetic leaflets are assessed carefully to ensure unhindered mobility. The aortotomy is closed, and the aortic clamp is removed. The patient is weaned from cardiopulmonary bypass with mild inotropic support. Transesophageal echocardiography is used to assess prosthetic function. Modified ultrafiltration is performed before decannulation.
Hospital mortality after mechanical aortic valve replacement in children is 0% to 5%. Early complications include permanent heart block in 3% and acute endocarditis in 2% of these patients. Late complications relate primarily to anticoagulation. In reports of long-term follow-up, valve thrombosis occurs in 0% to 2% of patients. This sometimes can be managed pharmacologically with thrombolytic agents but frequently requires urgent surgical thrombectomy or valve replacement. Embolic events are reported in 2%, and significant bleeding episodes occur at a rate of 0.15% per patient year. Freedom from reintervention or re-replacement is approximately 86% at 20 years but is increased in younger patients having smaller prostheses.
Ross Procedure
Ross reported aortic valve replacement with a pulmonary autograft and allograft reconstruction of the right ventricular outflow tract in 1967. In the absence of significant size discrepancy or connective tissue disease, the Ross procedure is the preferred technique for aortic valve replacement in small children. In larger children, it is frequently preferred over mechanical prosthesis to avoid the need for anticoagulation.
The approach is via a median sternotomy. The venae cavae are cannulated individually, and the ascending aorta is cannulated distally. The aorta is clamped, and in the absence of significant aortic insufficiency, cardioplegia is infused in the aortic root. Cardioplegia may also be delivered retrograde through a coronary sinus catheter and by direct coronary ostial perfusion after an aortotomy. Additional cardioplegia is delivered every 30 minutes during the cross-clamp period. A left ventricular vent is placed through the left atrial appendage or the right superior pulmonary vein.
The aorta and the pulmonary trunk are separated, and the pulmonary trunk is opened transversely just proximal to the bifurcation. The pulmonary valve is inspected to identify any pathology that would preclude its use as an aortic valve replacement. Transection of the pulmonary trunk is then completed. A right-angle clamp is passed carefully through the leaflets into the right ventricular outflow tract, and a site for proximal transection is identified in the infundibular free wall approximately 5 mm below the level of the valve. A transverse infundibular incision is made and carried to the infundibular septum at each end. The infundibular septum is scored with a scalpel blade. A plane can be developed between the subconal muscle and the underlying interventricular septum. Dissection in this plane completes the harvest, and the autograft is stored in normal saline solution before implantation. The resulting posterior raw surface is cauterized. In their practice, the authors also apply a thin layer of biological sealant to ensure hemostasis. In dissecting the autograft, care must be taken at the leftward extent of the septal dissection to avoid injury to the first septal perforating branch of the left anterior descending coronary artery. The pulmonary artery is sized, and an appropriate allograft is thawed and prepared for reconstruction of the right ventricular outflow tract.
The aorta is transected just above the sinotubular junction. The aortic valve leaflets are excised, and the left and right coronary arteries are excised with a generous button of aortic wall. The autograft is oriented by alignment of the commissures.
A proximal anastomosis is then constructed. Interrupted simple braided sutures are used for smaller patients, although a continuous technique with polypropylene sutures can be used in larger children and teenagers. The coronary buttons are anastomosed to incisions in the respective autograft sinuses of Valsalva. The distal aortic anastomosis is constructed with a running polypropylene suture. Air is evacuated from the left side of the heart, and the aortic clamp is removed. Continuity between the right ventricle and the pulmonary artery is restored by interposing the previously thawed allograft in the reperfused, beating heart. The patient is weaned from cardiopulmonary bypass with mild inotropic support. Transesophageal echocardiography is used to assess autograft and allograft function as well as left ventricular wall motion. Modified ultrafiltration is performed before decannulation in children with an operative weight below 20 kg.
A proximal anastomosis is then constructed. Interrupted simple braided sutures are used for smaller patients, although a continuous technique with polypropylene sutures can be used in larger children and teenagers. The coronary buttons are anastomosed to incisions in the respective autograft sinuses of Valsalva. The distal aortic anastomosis is constructed with a running polypropylene suture. Air is evacuated from the left side of the heart, and the aortic clamp is removed. Continuity between the right ventricle and the pulmonary artery is restored by interposing the previously thawed allograft in the reperfused, beating heart. The patient is weaned from cardiopulmonary bypass with mild inotropic support. Transesophageal echocardiography is used to assess autograft and allograft function as well as left ventricular wall motion. Modified ultrafiltration is performed before decannulation in children with an operative weight below 20 kg.
The results of the Ross procedure are reasonable in carefully selected pediatric patients. Early mortality is 0% to 6%, occurring primarily in infants under 5 months of age. However, patients with concomitant mitral valve disease or aortic arch hypoplasia, even when judged to have adequate biventricular physiology, fare poorly with the Ross operation. A recent paper by Shinkawa and colleagues from the University of Michigan reported a 36% early mortality in this subgroup. Similarly, Hickey and colleagues reported a 31% early mortality rate in infants younger than 3 months, and an actuarial 1-year survival <50% for neonates. Complications occur infrequently and include bleeding, arrhythmia, heart block, and stroke. Actuarial survival for all patients is 84% at 1 year and 77% at 5 years. Aortic root dilatation is common late after the Ross procedure in children and warrants careful echocardiographic follow-up.
Konno Aortoventriculoplasty
Annular enlargement is required in children with small aortic annular size requiring aortic valve replacement. Nicks and colleagues and Manougian and Seybold-Epting described techniques for posterior annular enlargement that have been used successfully in adults. However, the resulting increase in annular size is generally inadequate to allow insertion of even a small prosthetic valve in small children. Konno and coworkers described a technique of anterior enlargement that more effectively increases annular size and relieves coexistent subvalvar stenosis.
The approach is via a median sternotomy. The venae cavae are cannulated individually, and the ascending aorta is cannulated distally. The aorta is clamped, and in the absence of significant aortic insufficiency, cardioplegia is infused in the aortic root. Additional cardioplegia is delivered every 30 minutes during the cross-clamp period. A left ventricular vent is placed through the left atrial appendage or the right superior pulmonary vein.
A vertical aortotomy is made and is carried onto the right ventricular outflow tract well to the left of the origin of the right coronary artery. Care is taken to avoid injury to the pulmonary valve. The aortic valve leaflets are excised, allowing visualization of the left and right ventricular aspects of the infundibular septum. An incision is made across the aortic annulus into the infundibular septum. Injury to the conduction tissue is avoided by placing this incision to the left of Lancisi muscle. A diamond-shaped patch of Dacron is fashioned, and the inferior portion is sutured to the edges of the septal incision with interrupted pledgeted mattress sutures. An appropriately sized prosthetic valve is then inserted as was described for aortic valve replacement. Anteriorly, the valve sutures are passed through the prosthetic patch. The superior portion of the patch is used to close the ascending aorta. The right ventricular free wall is enlarged with a patch of bovine pericardium. Air is evacuated from the left side of the heart, and the aortic clamp is removed. The patient is weaned from cardiopulmonary bypass with mild inotropic support. Transesophageal echocardiography is used to assess prosthetic function, patch leaks, and left ventricular wall motion.
A modification of this technique can be used for annular enlargement in conjunction with the Ross procedure. A Ross-Konno is an ideal procedure in infants or children under 2 years of age, whose size precludes implantation of at least a 21-mm prosthesis or larger. The pulmonary autograft is harvested with a triangular portion of right ventricular free wall that is used as the septal patch.
In light of the complex form of LVOTO seen in pediatric patients who require the Konno or the Ross-Konno procedure, early and late results are quite good. On average, the annulus is enlarged to twice the original size, often allowing insertion of an adult-sized prosthesis (23 or 25 mm) in most patients over 3 years of age. Operative mortality for the Konno procedure with prosthetic valve replacement is 5% to 15%. Ten-year actuarial survival is 92%. Ten-year freedom from reoperation with a mechanical prosthesis is 80% to 89%. The linearized rate of reoperation is approximately 3.9% per year and is increased among patients undergoing a Konno to correct aortic valve pathology in conjunction with annular hypoplasia. An important and often underappreciated complication of the Konno procedure (with or without concomitant Ross operation) is pulmonary regurgitation, which occurs at a cumulative incidence of 10% at 16 years postoperatively. Operative mortality for the Ross-Konno procedure is 0% to 7% even in children under 1 year of age. Postoperative complications include bleeding, arrhythmia, heart block, and left ventricular dysfunction. No permanent effects on ventricular function are present at long-term follow-up.
SUBVALVAR AORTIC STENOSIS
Subaortic stenosis in children results from either a discrete fibrous membrane or, less commonly, diffuse, fibromuscular tunnellike stenosis (Fig. 84.4). The discrete subaortic membrane is probably an acquired lesion that is rarely seen in infants, albeit with “anatomic precursors.” Often, a ring of fibrous tissue is present that is adherent to the septum anteriorly, extending posteriorly to the right and left fibrous trigones and to the anterior mitral leaflet. Associated subaortic anomalies are present in 31% of these patients, including anomalous
septal insertion of the mitral valve, accessory mitral valve tissue, anomalous papillary muscles, and anomalous muscular bands. In addition to outflow obstruction, aortic insufficiency develops in more than 50% of these patients from turbulenceinduced leaflet deformity or direct attachment of the membrane to the aortic leaflets.
septal insertion of the mitral valve, accessory mitral valve tissue, anomalous papillary muscles, and anomalous muscular bands. In addition to outflow obstruction, aortic insufficiency develops in more than 50% of these patients from turbulenceinduced leaflet deformity or direct attachment of the membrane to the aortic leaflets.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree