Epidemiologic case definition (classic clinical criteria)
Fever of at least 5 days’ duration
Presence of at least four of the following principal features:
Changes in extremities
Polymorphous exanthem
Bilateral conjunctival injection
Changes in the lips and oral cavity
Cervical lymphadenopathy
Exclusion of other diseases with similar findings (Table 22.2)
Other clinical and laboratory findings
Cardiovascular findings
Congestive heart failure, myocarditis, pericarditis, or valvular regurgitation.
Coronary artery abnormalities
Aneurysms of medium-sized noncoronary arteries
Raynaud’s phenomenon
Peripheral gangrene
Musculoskeletal system
Arthritis, arthralgia
Gastrointestinal tract
Diarrhea, vomiting, abdominal pain
Hepatic dysfunction
Hydrops of the gallbladder
Respiratory tract
Pulmonary nodules and interstitial infiltrates
Pleural effusion
Central nervous system
Extreme irritability
Aseptic meningitis
Peripheral facial nerve palsy
Sensorineuronal hearing loss
Genitourinary system
Urethritis/meatitis
Testicular swelling
Other Findings
Erythema and induration at Bacille Calmette-Guerin (BCG) inoculation site
Anterior uveitis
Desquamating rash in groin
Laboratory findings in acute Kawasaki disease
Neutrophilia with immature forms
Elevated erythrocyte sedimentation rate
Elevated C-reactive protein
Elevated serum a-l-antitrypsin
Anemia
Abnormal plasma lipids
Hypoalbuminemia
Thrombocytosis after first week
Sterile pyuria
Elevated serum transaminases
Pleocytosis of CSF
Leukocytosis in synovial fluid
Cardiovascular Spectrum
The most important complication of KD is development of coronary artery aneurysms, which can cause angina, myocardial infarction, and sudden death. In the current era, if IVIG treatment is administered within 10 days of illness onset, coronary aneurysms are found in fewer than 5 % of children and giant aneurysms, in fewer than 1 % [56]. Other complications include peripheral artery aneurysms, which occur only among patients with giant coronary artery aneurysms, myocarditis with diminished left ventricular function, mitral and, less often, aortic regurgitation, and pericardial effusion.
Myocardial Infarction and Death
The principal cause of death in KD is acute myocardial infarction (MI). In a nationwide survey in Japan, Kato and colleagues analyzed 195 KD patients with MIs [57]. They found that the relative risk of MI was highest in the first year after KD. However, MI can occur many years later, particular as coronary artery stenoses worsen. Primary manifestations in children include shock symptoms, pallor, unrest, vomiting, and abdominal pain. Chest pain was more frequently reported in children older than 4 years of age. Asymptomatic MIs were seen in 37 % of patients. Twenty-two percent with MIs died; 16 % of the survivors of a first attack had a second MI. Fatalities were 63 % in a second attack and 83 % in a third attack. From review of the coronary angiograms of patients with MIs, most who died had obstructions in the left main coronary artery or in both the right coronary artery and the left anterior descending artery. In survivors, single-vessel obstruction, particularly in the right coronary artery, was frequently recognized.
Early recognition and treatment of MIs are a challenge in children. Recurrence of MI is observed in about 20 % of children who have had a previous MI. Because the mortality of recurrent MI is high, vigilant follow-up is needed for such patients. Patients with complications after MI, such as ventricular aneurysm, papillary muscle dysfunction, heart failure, severe arrhythmias, and postinfarction angina, are managed by medical or surgical approaches.
Myocardial infarction can also occur in adults, some of whom had “missed” KD in childhood. A recent study in San Diego County observed that 5 % of myocardial infarctions in individuals under age 40 years were caused by a past history of KD [58].
Systemic Artery Involvement
Coronary artery lesions account for almost all morbidity and mortality in KD patients. However, patient with giant coronary aneurysms may also have associated peripheral artery aneurysms. Indeed, aneurysms in other arteries were observed in 1.0 % of the patients in the natural history study of Kato and colleagues [59]. His angiographic study in 22 patients with systemic artery aneurysms demonstrated that systemic artery aneurysms were seen in axillary arteries in 18 cases, common iliac arteries in 16, internal iliac arteries in 12, renal arteries in six, mesenteric arteries in two, and internal thoracic arteries in two, respectively. One patient had large common iliac artery aneurysms associated with dilatation of aorta. Although the prognosis of systemic artery aneurysms is generally favorable, renovascular hypertension may develop in a patient having a renal artery lesion, and lesions in the internal mammary arteries may complicate the choice of graft in coronary bypass surgery. Gangrene of the extremities has been reported as a rare complication of KD [60].
Valvular Heart Disease, Myocarditis, and Pericarditis
Mitral regurgitation, generally of mild severity, is seen by echocardiography in approximately 25 % of children with acute KD [61]. The incidence of this valvulitis declines in the convalescent phase of the disease, and late mitral regurgitation is seen primarily in patients with ischemic disease affecting papillary muscle function [62]. Aortic regurgitation by echocardiography is virtually always mild and is seen in 1 % of patients over the first 5 weeks of illness [61]. Progression to severe regurgitation over several years has been reported in some patients [63]. Aortic root dilation is a recognized complication of KD that can persist, but is usually mild [64]. Pericarditis greater than 1 mm by echocardiography occurs in fewer than 5 % of children over the first 5 weeks of illness [61]; massive pericardial effusion and cardiac tamponade are rare. There have been no reports on its progression to chronic or constrictive pericarditis. Diminished left ventricular function is observed in approximately 20 % of patients in the acute phase, especially in the first and second weeks of illness [61]. Gallop rhythms, distant heart sounds, ST-T segment changes, and decreased voltage of R waves on electrocardiogram (ECG) may suggest the presence of myocarditis. Systolic function virtually always recovers to normal in the absence of ischemic heart disease [61, 65]. Diastolic dysfunction has been noted in acute KD, but long-term diastolic abnormalities are found predominantly in patients with coronary aneurysms [66].
Diagnostic Techniques
Coronary artery lesions in the acute stage of KD are detected by 2D echocardiography using standard views [56]. Indeed, echocardiography is highly sensitive and specific for the diagnosis of dilated lesions and even the presence of thrombus in the proximal coronary arteries. However, echocardiography is less reliable for detection of distal aneurysms or stenotic lesions. Reliable coronary imaging of young children (e.g. younger than age 2 years) generally requires the use of sedation. The diagnosis of coronary artery aneurysms may be made by two different sets of criteria. First, the 1984 Japanese Ministry of Health criteria use absolute coronary dimensions for the diagnosis of coronary aneurysms as follows: coronary arteries with lumen diameter greater than 3 mm in children younger than 5 years or greater than 4 mm in those older than age 5; lumen diameter 1.5 times the size of an adjacent segment; or irregular lumen [67]. However, this approach may underestimate the true incidence of coronary artery dilatation, so coronary artery dimensions adjusted for body surface area, i.e., so-called coronary artery z scores, have come into common use [68, 69]. Figure 22.1 depicts normal coronary dimensions according to body surface area.
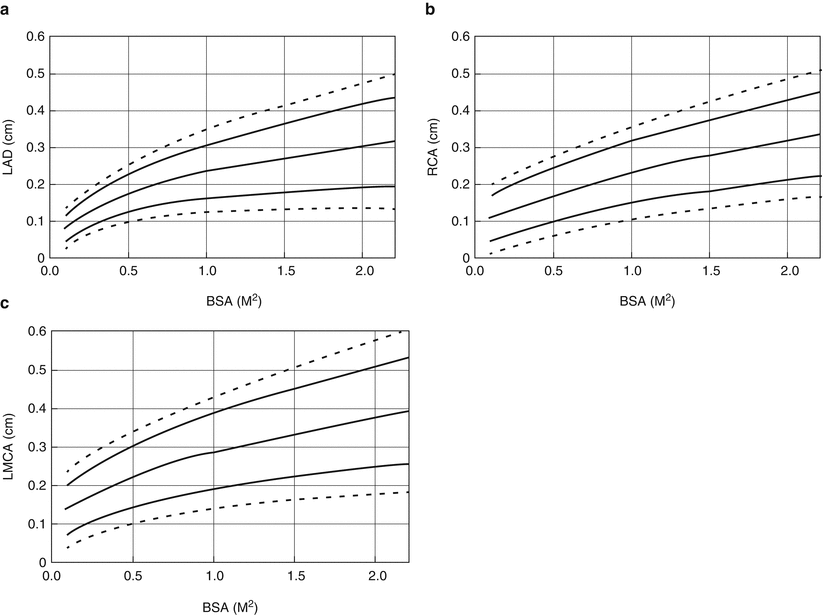

Fig. 22.1
Mean and prediction limits for 2 and 3 standard deviations for size of left anterior descending coronary artery (LAD, Panel a); proximal right coronary artery (RCA, Panel b), and left main coronary artery (LMCA, Panel c) according to body surface area for children younger than age 18 years. Left main coronary artery z scores should not be based on the dimension at the orifice and its immediate vicinity; enlargement of the LMCA secondary to KD is usually associated with ectasia of the LAD, LCX, or both (From AHA Scientific Statement – Newburger et al. [56]. Reprinted with permission from American Heart Association)
The method for evaluation by echocardiography is well described in the American Heart Association scientific statement on KD published in 2004 [56] and is summarized in Table 22.2. Aneurysms are classified as saccular if the axial and lateral diameters are nearly equal or as fusiform if symmetric dilatation with gradual proximal and distal tapering is seen. If the coronary artery diameter looks larger than normal without segmental dilatation, the coronary artery is considered ectatic. Echocardiography is advised at the time of diagnosis, and at 2 and 6 weeks after illness onset. For patients with risk factors for aneurysms, such as persistent or recrudescent fever after treatment with intravenous immunoglobulin (IVIG), age less than 6 months, or coronary dilation at baseline, echocardiography should be performed more frequently. In early literature upon which much of the natural history is based, the sizes of coronary artery aneurysms are classified as small (<5 mm internal diameter), medium (5–8 mm) and giant or large (>8 mm). Some investigators classify aneurysms according to z score, with giant aneurysms defined as those with a maximum internal diameter of ≥10 [70].
Table 22.2
Echocardiographic views of coronary arteries in patients with Kawasaki disease
Left main coronary artery: precordial short axis at level of aortic valve; precordial long axis of left ventricle (superior tangential); subcostal left ventricular long axis |
Left anterior descending coronary artery: precordial short axis at level of aortic valve; precordial superior tangential long axis of left ventricle; precordial short axis of left ventricle |
Left circumflex: Precordial short axis at level of aortic valve; apical four-chamber |
Right coronary artery, proximal segment: precordial short axis at level of aortic valve; precordial long axis (inferior tangential) of left ventricle; subcostal coronal projection of right ventricular outflow tract; subcostal short axis at level of atrioventricular groove |
Right coronary artery, middle segment: precordial long axis of left ventricle (inferior tangential); apical four-chamber; subcostal left ventricular long axis; subcostal short axis at level of atrioventricular groove |
Right coronary artery, distal segment: apical four-chamber (inferior); subcostal atrial long axis (inferior) |
Posterior descending coronary artery: apical four-chamber (inferior); subcostal atrial long axis (inferior) |
Other Noninvasive Techniques
When echocardiographic images are inadequate, for example in the larger child or adult, magnetic resonance imaging (MRI) and ultrafast computed tomography (CT) are useful noninvasive techniques for evaluation of coronary artery architecture [71–75]. Coronary images are superior with CT, but MRA has the advantage, especially in young individuals, of avoiding nonionizing radiation. In addition, dobutamine or adenosine stress testing can be done with MRI in the patient who is too young to cooperate by exercising on a bicycle or treadmill.
Coronary Angiography and Heart Catheterization
Selective coronary angiography (CAG) is the “gold standard” for evaluation of the presence and severity of coronary arterial abnormalities in KD patients. Indications for CAG include the presence of significant coronary aneurysms by 6–12 months after illness onset in patients when CT or MRI cannot provide high-quality information at lower risk; evidence of ischemic myocardial disease or angina requiring revascularization; or need to delineate precise coronary architecture for therapeutic management decisions, for example the type of anti-thrombotic therapy. If the patient has severe coronary artery lesions, other systemic vascular involvement such as the auxiliary, iliac, renal, or intrathoracic arteries aneurysms should also be evaluated. Follow-up coronary angiography is often needed for evaluation of stenotic or obstructive lesions of coronary arteries and for evaluation of collateral circulation late after KD because the extreme coronary calcification can limit the utility of CTA. Intravascular ultrasound performed during cardiac catheterization can provide information about myointimal thickening, generally present in arterial segments that were larger than 4 mm early in the disease [76], and measurement of coronary flow reserve may provide guidance for decisions about revascularization.
Evaluation for Myocardial Ischemia
Because the morbidity and mortality of this disease primarily depend on the extent of associated coronary artery disease, it is particularly important to assess accurately the presence of myocardial ischemia during follow-up. The choice of test modality for evaluation of inducible myocardial ischemia in children depends upon the age of the child and his/her ability to exercise, as well as institutional experience. When feasible, exercise stress testing is preferable to pharmacologic stress testing because it mimics the physiology of daily activities, and stress-echocardiography is preferable to nuclear or PET stress testing to diminish exposure to ionizing radiation. In children who are too young to exercise, dobutamine or adenosine stress MRI or other pharmacologic stress testing modalities are useful.
Evidence-Based Treatment and Management
Treatment of Acute KD
Intravenous Immunoglobulin (IVIG)
The standard of care for treatment of acute KD is IVIG at a dose of 2 g/kg over 8–12 h [56]. This treatment should be started within 10 days of illness, and if possible, within 7 days. It is uncertain whether very early treatment, that is, within 4 days of illness, is more effective [77]. In a meta-analysis, IVIG had a dose-dependent effect, with higher doses in single infusions being more effective than smaller doses or more protracted courses [78]. The mechanism(s) of action of IVIG is uncertain. Possible Fc-mediated mechanisms include down-regulation of inflammatory cytokines; modulation of T/B-cell functions through binding to lymphocyte Fc receptors; and inhibition of activated complement to targets, such as vascular endothelium. Possible Fab-mediated mechanisms entail the neutralization of microbial toxins, antibodies against cytokines and anti-idiotypic reaction against autoantibodies.
Aspirin
Intravenous immunoglobulin is generally administered with aspirin, initially at antipyretic dosages (80 mg/kg/day divided q6h), then lowered to 3–5 mg/kg/day for anti-platelet effect after the child has been afebrile for 48 h [56]. In Japan, a lower dose of aspirin, 30 mg/kg, is generally prescribed in the acute stage and reduced to 5 mg/kg in convalescent stage. Aspirin is discontinued after 6 weeks in children without evidence of coronary aneurysms. Of note, aspirin does not prevent the occurrence of coronary aneurysms.
Treatment of the IVIG-Resistant Patient
Among patients with persistent or recrudescent fever without other cause (i.e., presumed secondary to KD) at least 36 h after initial IVIG infusion, IVIG retreatment is given at a dose of 2 g/kg over 8–10 h [56]. A course of corticosteroids may also be helpful [79]. Other therapies that may be useful in the IVIG-resistant patient include infliximab [80, 81], cyclosporine [82], cyclophosphamide [83], ulinastatin (not available in the US) [84], methotrexate [85], and plasmapheresis [86]. In a small series, abciximab appeared to be associated with improved regression of coronary aneurysms [87].
Adjunctive Agents Used in Primary Treatment
In Japan, where risk scores have excellent predictive value for the likelihood of aneurysm development, Kobayashi and colleagues have demonstrated that addition of corticosteroids to conventional therapy with IVIG and aspirin lowers the risk of coronary aneurysms and shortens the duration of inflammation among high-risk children [88]. A randomized trial of pulsed-dose corticosteroids for primary treatment of KD in the United States was negative, but the study population had standard rather than high risk for aneurysm development [89]. The use of TNF-alpha blockers infliximab and etanercept for primary therapy of KD are currently under study.
Long-Term Management
Long-term management of patients with KD depends on the degree of coronary arterial involvement. Table 22.3 summarizes the 2004 American Heart Association recommendations for anti-thrombotic management, frequency of follow-up, types of testing, and exercise recommendations in long-term management of patients with this disease.
Table 22.3
Risk stratification
Risk level | Pharmacologic therapy | Physical activity | Follow-up and diagnostic testing | Invasive testing |
|---|---|---|---|---|
I (no coronary artery changes at any stage of illness) | None beyond initial 6–8 weeks | No restrictions beyond initial 6–8 weeks | Cardiovascular risk assessment and counseling at 5-year intervals | None recommended |
II (transient coronary artery ectasia that disappears within initial 6–8 weeks) | None beyond initial 6–8 weeks | No restrictions beyond initial 6–8 weeks | Cardiovascular risk assessment and counseling at 3- to 5-year intervals | None recommended |
III (small to medium solitary coronary artery aneurysm) | Low-dose aspirin (3 to 5 mg/kg aspirin per day), at least until aneurysm regression is documented. | For patients in first decade of life, no restriction beyond initial 6–8 weeks. For second decade, physical activity guided by stress testing every other year. Contact or high-impact sports discouraged for patients on anti-platelet agents. | Annual cardiology follow-up with echocardiogram and ECG, combined with cardiovascular risk assessment and counseling. Stress testing with radioisotope perfusion scan or stress echocardiogram every other year. | Angiography, if non-invasive test suggests ischemia |
IVa (one or more large or giant coronary artery aneurysms), or IVb (multiple or complex aneurysms, without obstruction) | Long-term anti-platelet therapy and warfarin (target INR 2.0–2.5) or LMW heparin (target: antifactor Xa level 0.5–1.0 units/mL) should be combined in giant aneurysms. | Contact or high-impact sports, isometrics, and weight training should be avoided because of the risk of bleeding. Other physical activity recommendations guided by outcome of stress testing or myocardial perfusion scan. | Biannual follow-up with echocardiogram + ECG. Annual pharmacological or exercise stress testing. | Initial angiography at 6–12 months. Repeated angiography if non-invasive test, clinical or laboratory findings suggest ischemia. Elective repeated angiography under some circumstances (see text) |
V (coronary artery obstruction) | Long-term low-dose aspirin. Warfarin or LMW heparin if giant aneurysm persists. Use of beta blockers should be considered to reduce myocardial oxygen consumption | Contact or high-impact sports, isometrics, and weight training should be avoided because of the risk of bleeding. Other physical activity recommendations guided by outcome of stress testing or myocardial perfusion scan. | Biannual follow-up with echocardiogram and ECG. Annual pharmacological or exercise stress testing. | Angiography is recommended to address therapeutic options. |
Anti-thrombotic Management
Coronary thrombosis is the primary mode of demise for children with coronary aneurysms after KD. There are no randomized trials to guide optimal management, so recommendation for treatment is based upon consensus of experts, small observational studies, and adult experience [56]. All children with coronary aneurysms are treated with anti-platelet dose aspirin. In patients with giant aneurysms, who are at the highest risk for coronary thrombosis, anticoagulation with warfarin or low-molecular-weight heparin is added to aspirin [90–92]. Among children with aneurysm size just below the threshold for giant, or among patients with giant aneurysms in whom anti-coagulation cannot be safely managed by a family, a thienopyridine is often added to aspirin. Finally, in children who have required thrombolysis for coronary thrombosis, triple therapy with anti-coagulation, aspirin, and a thienopyridine may be used for a short time, balancing the risk of recurrent thrombosis with that of bleeding.
Coronary Thrombosis and Myocardial Infarction (MI)
Reports of treatment of acute myocardial infarction in KD patients are few [93], and hence the strategies for acute revascularization are based upon those used in adults. The optimal therapy is prompt restoration of perfusion by interventional catheterization [94]. However, mechanical restoration of flow is not feasible in the youngest children, for whom thrombolytic therapy with t-PA (together with aspirin and low-dose heparin) is standard therapy [56]. Occasionally, a coronary thrombus in a proximal giant aneurysm is noted on routine echocardiographic surveillance. When a non-occlusive mural thrombus is detected, treatment with t-PA may be given in the absence of myocardial ischemia because of the expectation that the thrombus will continue to grow and become occlusive. Some physicians administer abciximab instead of t-PA or together with half-dose t-PA to such patients to prevent clot extension.
Pregnancy
Women with giant aneurysms are managed in a fashion similar to that used in women with mechanical heart valves and should have reproductive counseling beginning in the teenage years [95].
Coronary Revascularization
Both coronary artery bypass surgery and percutaneous coronary intervention have been performed in KD patients [96, 97]. No randomized clinical trials have been performed either to assess indications for these procedures or to compare outcomes between surgical and catheter techniques. A Japanese survey has suggested that these treatment modalities result in similar rates of death or myocardial infarction; however, reinterventions were more commonly performed after initial percutaneous intervention [98]. Not surprisingly, graft failure was most common among patients who underwent revascularization without having had evidence of myocardial ischemia.
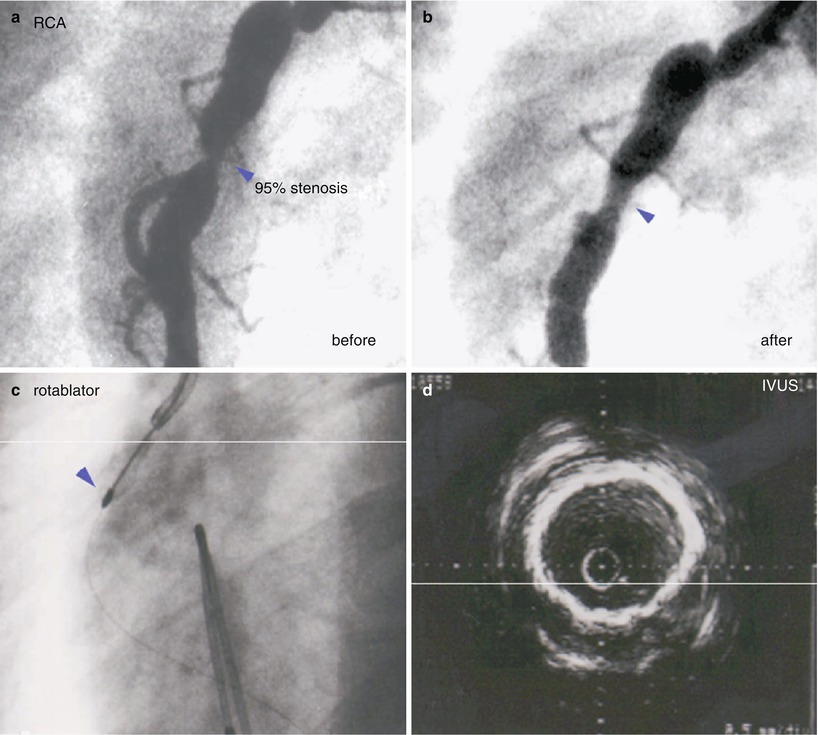
Percutaneous Coronary Intervention
Guidelines for catheter intervention in KD patients have been published by the Japanese KD Research Committee [99] and include the presence of ischemic symptoms, inducible myocardial ischemia on stress testing, or more than 75 % stenosis of the left anterior descending coronary artery. Children with severe left ventricular dysfunction or complex coronary lesions with multiple, ostial or long-segment stenosis are not considered to be candidates for PCI in these guidelines. Percutaneous procedures should be performed by adult interventional cardiologists, with support from a pediatric team if needed. Because coronary artery calcification is common and worsens with time, rotational ablation and stent placement are preferred to percutaneous transluminary angioplasty; data are limited on use of drug-eluting stents (Fig. 22.2).