Cardiac
MRI for the evaluation of ischemic heart disease has tremendous potential because of its high
spatial resolution and image
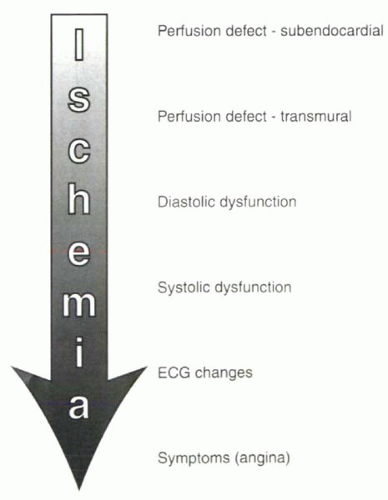
contrast. In considering the different diagnostic strategies that can be used, it is helpful to review the progression of detectable abnormalities in ischemic heart disease, shown in
Figure III7-1. The two main diagnostic strategies measure changes in either perfusion or ventricular wall motion under pharmacologic stress compared with rest. Although qualitative perfusion imaging is more sensitive for early ischemic change, it is also less specific than wall motion studies and may be less accurate in patients with global ischemia.
The
MR imaging techniques and methods for pharmacologic stress vary considerably across various laboratories. Validation studies of both
MR approaches are still being performed, and, in the context of alternative techniques such as stress
ECG, SPECT, echocardiography, and PET, the role of
MR in the evaluation of patients with ischemic heart disease remains to be defined fully. Some basic principles of stress perfusion and wall motion studies are outlined in this chapter, with the caveat that these methods are continuing to be refined.
PHARMACOLOGIC STRESS IN THE MRI SETTING
For the evaluation of ischemic heart disease, all diagnostic techniques are performed with cardiac stress. Stress can be achieved with exercise or with pharmacologic agents. In general, for exercise and sympathomimetic
stress, the goal is to achieve a submaximal
target heart rate that depends on age:
While ergonomic stress is the most physiologic, exercising in the magnet bore is not generally practical. Therefore, cardiac
MR studies typically are performed with pharmacologic agents using one of two approaches: (a)
dobutamine, a catecholamine that causes increased contractility and oxygen consumption, or (b) either
adenosine or dipyridamole, both vasodilators that cause hyperemia. In both cases, changes that are observed are different for regions of myocardium that are supplied by diseased coronary arteries compared with those supplied by normal coronary arteries.
For all stress testing, careful monitoring of patients in the
MR setting is vital. A physician experienced in cardiac resuscitation should be present for all examinations. There must be a continuous communication between the subject and operators to ensure prompt attention to symptoms. Monitoring should include frequent blood pressure measurements, continuous pulse oximetry, and continous single-lead
ECG tracings for heart rate and rhythm. Although the detection of ST segment changes is problematic in the
MR scanner because of the
magnetohydrodynamic effect (see
Chapter III-1), wall motion abnormalities generally precede
ECG changes. With real-time
cine MRI early detection of ischemia is possible.
Dobutamine
Dobutamine is a synthetic catecholamine with potent β
1-receptor and mild α
1– and β
2-receptor agonist activity. The effect of dobutamine depends on the dose administered. At low doses (≤ 10 μg/kg body weight/min), dobutamine causes increased contractility. The recovery of wall motion with low-dose dobutamine is one technique for assessing cardiac viability in the setting of resting hypokinesis or akinesis. At high doses of dobutamine, increased heart rate and contractility cause an increase in oxygen consumption. Areas supplied by significantly diseased coronaries develop wall motion abnormalities under these conditions. The stress protocol is based on that used for dobutamine echocardiography (
Table III7-1).
Beta-blockers reduce the effects of dobutamine and generally are withheld for 24-48 hours prior to the examination. Similarly, calcium antagonists and nitrates are discontinued for 24 hours prior to the study.
Contraindications for the administration of dobutamine include acute coronary syndrome, severe aortic stenosis, hypertrophic obstructive cardiomyopathy, uncontrolled hypertension, uncontrolled atrial fibrillation, uncontrolled heart failure, and known severe ventricular arrhythmias.
As shown in
Table III7-1, the conventional protocol involves incremental increases in dobutamine doses in steps of 10 μg/min/kg body weight, up to a maximum dose of 40 (or even 50) μg/min/kg. If
target heart rate is not reached, additional atropine can be administered at doses of 0.25 mg, up to 1-2 mg total, during the ongoing infusion of
dobutamine. Criteria for termination of the study are shown in
Table III7-2 and include the development of signs and symptoms of ischemia—including wall motion abnormalities in at least two adjacent ventricular segments or anginal symptoms.
Side effects of the dobutamine can be reversed with a β-blocker such as intravenous esmolol or metoprolol if signs and symptoms do not resolve after infusion is stopped. The half-life of dobutamine is short—approximately 2 min. In patients who develop angina, sublingual nitroglycerin can also be used.
Dipyridamole and Adenosine
Dipyridamole (Persantine) and
adenosine have almost identical vasodilatory effects.
Adenosine is a naturally occuring vasodilator. Dipyridamole blocks the cellular uptake and metabolism of
adenosine and consequently also causes vasodilation. With both agents, a four- to five-fold hyperemia is seen in normal myocardial territories but not in regions subtended by stenotic coronary arteries. With significant coronary artery disease,
dipyridamole and
adenosine cause a steal phenomenon, and the differential perfusion changes with vasodilation are used to diagnose ischemic heart disease.
Contraindications include asthma, high-grade atrioventricular block, sinus arrhythmia, aortic or mitral valvular stenosis, and carotid artery stenosis. Medications that contain aminophylline, theophylline, and other xanthines (such as caffeinated products) are withheld for 12-24 hours before the study.
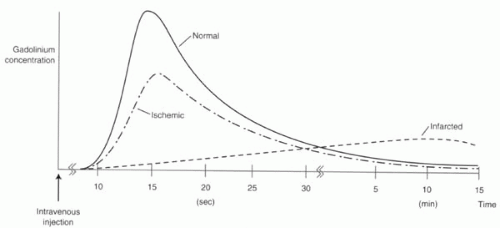
Assessment of perfusion is performed using first-pass gadolinium-enhanced perfusion. The standard dosing regimens for the two vasodilatory agents are shown in
Table III7-3.
Reasons for stopping the infusion of these agents include bronchospasm, ventricular arrhythmias, and the onset of second- or third-degree atrioventricular block and bradycardia. Low-workload exercise (such as handgripping exercises) have been shown to reduce the side effects associated with the vasodilators. Intravenous aminophylline is an antidote to both
adenosine and dipyridamole and should be available for immediate administration. The plasma half-life of
adenosine is less than 10
sec. For intravenous
dipyridamole, it is 5 min.
Pharmacologic Stress in the Diagnosis of Ischemic Heart Disease
Both categories of pharmacologic agents—sympathomimetics such as
dobutamine or vasodilators such as
adenosine or
dipyridamole—improve the diagnosis of ischemic heart disease by accentuating differences between territories supplied by normal coronary arteries and those that receive inadequate blood supply. In the clinical setting, the most common imaging strategies for detecting these differences are stress radionuclide perfusion examination and stress echocardiography. The
MR tools recapitulate these approaches.
MRI can be used either to measure perfusion, typically with first-pass gadolinium-enhanced Tl-weighted imaging, or to detect changes in wall motion with fast cine gradient echo imaging. Each class of stressor agent can be used with either
MRI approach. However, most commonly, perfusion imaging is performed with vasodilators and contractility studies are performed with dobutamine. Whether to use perfusion or contractility methods remains the subject of active research. Some considerations include the extent of coronary disease, tradeoffs between sensitivity and specificity, tolerance by patients, cost, and ease of administration.