High-risk category
Neutropenia (neutrophil count <500/mm3)
Allogeneic bone marrow transplantation
Hematological malignancy
Intermediate-risk category
Prolonged treatment with corticosteroids before admission to the ICU
Autologous bone marrow transplantation
Chronic obstructive pulmonary disease stage III or IV
Liver cirrhosis Child C
Solid-organ cancer
ARDS treated with >80 mg methylprednisolone per day (for at least 3 days)
Lung transplantation
Systemic diseases such Wegener’s granulomatosis and microscopic polyangiitis
HIV infection
Low-risk category
Severe burns
Prolonged stay in the ICU (>21 days)
Chronic obstructive pulmonary disease stage II
Other solid-organ transplant recipients (heart, kidney, or liver)
Post-cardiac surgery status
Malnutrition (BMI <18)
Steroid treatment with a duration of less than 3 days
Various factors, including the prolonged use of antibiotics and the use of central venous catheters and/or mechanical ventilation, adversely affect the defense systems of previously healthy individuals (Stevens et al. 2000). Although these factors are present in most ICU patients, many of these patients do not develop IA. One of the intriguing hypotheses for immunosuppression in the apparently immunocompetent patient with multiple-organ dysfunction relates to the biphasic response to sepsis (Hartemink et al. 2003). The initial hyperinflammatory phase is followed by relative immunoparalysis (Kox et al. 2000). This latter process is characterized by neutrophil deactivation, and it may put the patient at risk of developing opportunistic infections such as IA. Additional epidemiological studies are warranted to better delineate this phase of immunoparalysis.
Patients in the ICU (medical and surgical) are often treated with steroids. Recent work concluded that the mortality rate is reduced if patients with septic shock who have adrenal dysfunction receive hydrocortisone for a 7-day period (Annane et al. 2002). However, in vitro pharmacological concentrations of hydrocortisone accelerate the growth of Aspergillus species (Lionakis and Kontoyiannis 2003). Clearly, high steroid intake diminishes both lines of cellular defense against IA (i.e., macrophages and neutrophils). This has been demonstrated in hematopoietic stem cell transplant recipients who received prolonged courses of steroids for the treatment of graft-versus-host disease (O’Donnell et al. 1994; Martino et al. 2002). Palmer et al. (1991) reported that the threshold steroid concentration varies according to the type of patient, and they emphasized that underlying lung disease is a risk factor for IA even when low doses of steroids are administered. Cases of IA have even been reported in association with inhaled steroids (Leav et al. 2000). Additional studies are needed to investigate whether administration of the 7-day course of hydrocortisone (200 mg/day) to patients with septic shock puts them at risk of developing IA, knowing that recognition of fungal infection may be delayed because the anti-inflammatory properties of steroids blunt the signs of infection (Graham and Tucker 1984). Recently, during the H1N influenza outbreak of 2009, 23 % of our patients with proven H1N1 developed probable or proven aspergillosis during the course of their illness. Almost all of them were treated with corticosteroids for the underlying acute respiratory distress syndrome (ARDS) caused by H1N1 (Wauters et al. 2012).
Two at-risk groups not included in the EORTC/MSG definitions stand out with regard to IA: patients with chronic obstructive pulmonary disease (COPD) and patients with cirrhosis. Patients with COPD have an increasingly recognized risk of developing IA and, in some institutions, cases of IA among patients with COPD outnumber those cases in “classic” patients. Bulpa et al. (2001) analyzed a group of 16 patients with COPD who had proven or probable IA and who required ICU admission. All patients were receiving steroid treatment. The outcome was invariably poor. This is in accordance with the findings of Rello et al. (1998), who described another 8 patients with COPD and IA, among whom the outcome was universally fatal.
Hepatic failure is generally not recognized as a risk factor for IA. A literature review revealed that 5 of 14 previously reported cases of IA in seemingly immunocompetent hosts were associated with liver disease (Ascah et al. 1984). Our study revealed three fatal cases of IA (Meersseman et al. 2004). Patients with cirrhosis experience depressed phagocytosis, which may increase their risk for severe infections (Bailey et al. 1976).
It is expected that new risk categories of IA will arise as new immunosuppressive agents, such as alemtuzumab and etanercept (a TNF-α blocker), are made available (Martin et al. 2006; Warris et al. 2001).
IV. Do Patients Acquire Aspergillosis in the ICU?
There are numerous sources of Aspergillus species for patients in the ICU (Patterson 2005). Some studies suggest that fungal colonization of the lungs is present before entry into the hospital (Lass-Flörl et al. 1999). It is believed that the primary ecological niche is decomposing material (Segal and Walsh 2006). However, aerosolized spores may become a potential source of infection through improperly cleaned ventilation systems, water systems, or even computer consoles (Warris and Verweij 2005). The use of high-efficiency particulate air filtration reduces the risk of IA but does not reduce it to zero, probably in part because patients may be colonized before admission to the ICU, and partly because of breaks in airflow (Munoz et al. 2004). Pittet et al. (1996) described two patients who developed fatal IA in the ICU. In retrospect, high concentrations of airborne Aspergillus spores could be found in close proximity to an air filter change in the ICU. An outbreak in a Spanish post-cardiac surgery unit was reported in 2012. The demonstration of matches between air and clinical genotypes reinforces the role of environmental air in the acquisition of aspergillosis. High-efficiency particulate air filters were not able to prevent the outbreak (Pelaez et al. 2012). Environmental monitoring of Aspergillus spores in the air is mandatory but, to our knowledge, a study of ventilators as a source of infection has not been undertaken. Of note, the development of IA depends on an interplay between the inoculating dose, the ability of the host to resist infection, and the virulence of the organism.
In the retrospective study performed in our unit, 63 of 102 patients (62 %) with a culture positive for Aspergillus species had received the positive culture result within 1 week after admission to the ICU. Almost all patients were undergoing mechanical ventilation, and the mean duration of ICU stay was 20days. Of the patients with proven cases, 18 of 26 (69 %) with an underlying hematological malignancy and 11 of 30 (37 %) without a malignancy had clinical evidence of IA at the time of admission to the ICU (Meersseman et al. 2004). However, there is no consensus about the incubation period and estimates range from 2 days to 3 months. Moreover, culture results and clinical evidence alone are not reliable predictors for invasive disease. The concept that the increasing fungal burden associated with specific ICU treatments for diseases other than IA (e.g., steroid therapy for septic shock) parallels the progression from subclinical to clinical aspergillosis needs to be explored using more secretion specimens as a modality for surveillance is an interesting topic for research in the ICU.
V. Disease Manifestations in the ICU
There are several manifestations of IA disease in the ICU. There are three types of pulmonary pathogen–host interactions (Soubani and Chandrasekar 2002). The most frequent interaction is colonization of the airways; this can be present in patients with defective mucociliary clearance and structural changes in the bronchial wall (Hope et al. 2005a). These changes are present in almost every patient who is undergoing mechanical ventilation, making them particularly susceptible to colonization. IA will not develop in these patients unless a critical level of immunodeficiency has been reached. The second type of interaction is “allergic” in nature and is beyond the scope of this review. The most relevant form of interaction for ICU physicians is the invasive disease that develops in persons with impaired immunity. The lungs and sinuses are implicated in >90 % of these cases (Patterson et al. 2000). The aggressive angioinvasive form of IA is frequently encountered in neutropenic patients, whereas cavitating infiltrates are observed most frequently in patients who are receiving steroids, patients with COPD, patients with cirrhosis, solid-organ transplant recipients, etc. (Denning et al. 2003). In lung transplant recipients, anastomotic infections are the most frequently occurring presentations (Nathan et al. 2000; Mehrad et al. 2001).
Other, rarer, presentations include endocarditis, wound infection, mediastinitis (after cardiac surgery), infection of vascular grafts, and osteomyelitis; these are occasionally a problem in immunocompromised patients or during epidemic outbreaks. A detailed description of all disease entities is beyond the scope of this article and was recently reviewed elsewhere (Pasqualotto and Denning 2006; Nunley et al. 2002). Infection of the central nervous system is frequently an ominous sign and may arise from hematogenous seeding (for which the lung is the most common primary site), from spread of the pathogen from the sinuses, or after neurosurgery (Lin et al. 2001; Marr et al. 2002).
The pathogenesis of IA in patients with steroid-associated immunosuppression differs greatly from that in neutropenic patients. Data demonstrate that pathologic lesions are often widespread and that death is related to a high fungal burden in neutropenic animals, whereas the pathogenesis in non-neutropenic, steroid-treated animals is driven by an adverse inflammatory host response that is frequently confined to the lungs, with a low fungal burden in the lung parenchyma and other organs (Balloy et al. 2005; Chamilos et al. 2006).
Clinical signs are usually nonspecific and do not necessarily differ from those for other causes of nosocomial pneumonia. In addition, critically ill patients with prolonged stays in the ICU often develop pulmonary infiltrates, atelectasis, and/or acute respiratory distress syndrome (ARDS), whereas patients with prior lung disease (e.g., COPD) may present with preexisting cavities noted by conventional chest radiography.
VI. Are the Available Diagnostic Tools Applicable to Patients in the ICU?
Making a timely diagnosis of IA in the ICU population is probably even more challenging than establishing an early diagnosis in patients with hematologic disease. Basically, this is because the index of suspicion is lower in the ICU population because most patients do not belong to one of the well-established risk groups. Moreover, the diagnostic tools were developed in hematology patients. In general, a diagnosis is made on the basis of a combination of compatible clinical findings, abnormal radiologic findings, and microbiologic confirmation or on the basis of histologic proof of tissue invasion by the fungus (Hope et al. 2005).
Over the past few years, lung computed tomography (CT) has become one of the most important diagnostic tools. Diagnostic signs of angioinvasive pulmonary mycosis – not only that due to Aspergillus species, but occasionally that due to Mucorales species – include single or multiple small nodules with the halo sign. It should be recognized that the utility of this sign has been evaluated almost exclusively in neutropenic patients (Caillot et al. 1997). In other groups, including ICU patients, similar CT findings are frequently absent, and if the signs are present, they are far less specific. Many ICU patients have nonspecific interfering radiologic abnormalities associated with atelectasis or ARDS (Figs. 10.1, 10.2, 10.3, and 10.4).
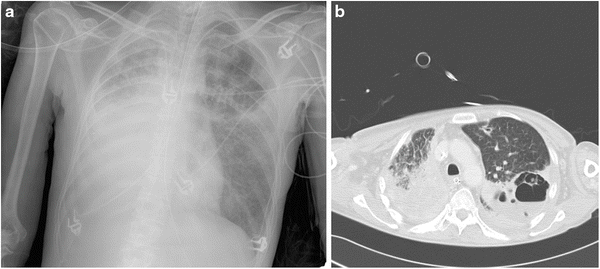
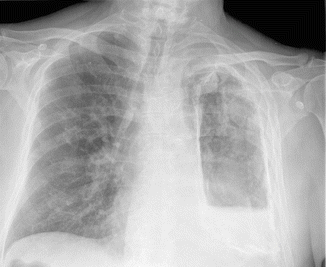
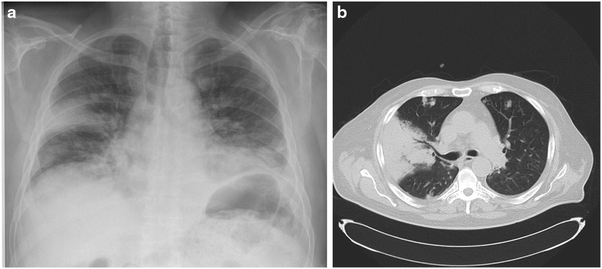
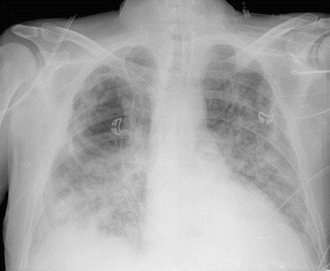

Fig. 10.1.
(a) Chest X-ray and (b) CT scan of a 42-year-old lady with adenocarcinoma of the right lung with adjacent malignant pleural effusion who was receiving chemotherapy (cisplatinum and gemcitabine) and who was admitted to the intensive care unit because of fever and new pulmonary infiltrates. No neutropenia. A large right-sided pleural effusion and a cavity in the upper zone of the left lung were seen in the chest X-ray. CT scan confirmed a complex cavitary lesion in the left lung and a pleural effusion around the right lung. Bronchoalveolar lavage (BAL) culture was positive for Haemophilus influenzae and negative for fungi. The results of a serum galactomannan test were negative but revealed a value of 6.2 ng/ml in the BAL fluid specimen. Despite administration of voriconazole, the patient died. Autopsy revealed invasive aspergillosis that was confined to the left lung. Residual malignant lesion was present in the right pleura and lung parenchyma

Fig. 10.2.
Chest radiograph of 72-year-old patient referred for pneumectomy. At the time of referral, he had major hemoptysis. He was known to have suffered from tuberculosis 20 years earlier. Chest X-ray pre-operatively shows a destroyed left lung, an air-fluid level with widespread pleural calcifications. Culture from a tracheal aspirate revealed Aspergillus fumigatus. GM value of a BAL specimen was 9.8 ng/ml. Serum galactomannan was below detection limit. No reactivation of tuberculosis was found. Patient was treated with voriconazole. Pneumectomy was uneventful. Biopsy of necrotic tissue per-operatively showed dichotomous branching hyphae, which turned out to be Aspergillus fumigatus upon culture

Fig. 10.3.
(a) Chest radiograph and (b) CT of a 65-year-old man who was diagnosed as having Wegener’s granulomatosis 1 month prior to presentation. Patient had renal involvement without pulmonary involvement initially. He was treated with high-dose steroids and cyclophosphamide. One month later, he developed fever, hemoptysis, and acute respiratory failure. He required mechanical ventilation. Chest X-ray shows bilateral patchy infiltrates with a large wedge-shaped lesion on the right side. CT scan showed a large triangled infiltrate in the right lung suggestive of pulmonary infarction. Widespread nodules are seen throughout both lungs. Bronchial culture for fungi remained negative, although BAL GM and serum GM values were both raised (8.4 and 3.2 ng/ml respectively). Patient died soon after admission to the ICU following ventricular fibrillation. Autopsy revealed disseminated aspergillosis (lung, thyroid gland, heart, adrenals, and spleen)

Fig. 10.4.
Chest radiograph (day 22 post-ICU admission) of a 52-year-old man with Child C liver cirrhosis. He was originally admitted for a spontaneous bacterial peritonitis. Blood cultures revealed Escherichia coli. He developed multiple organ failure. On day 20 post-admission to the ICU, he was still mechanically ventilated and developed new-onset fever and progressively increased oxygen requirements. Bronchial culture revealed Pseudomonas aeruginosa. Despite meropenem, he deteriorated. A chest X-ray showed bilateral infiltrates with the possibility of a cavity in the right lung. A CT scan was not feasible. A new bronchial culture obtained on day 23 revealed Aspergillus fumigatus. BAL GM was 3.1 ng/ml and serum GM was 0.7 ng/ml. Anidulafungin was started on day 25. No voriconazole IV was given because of severe renal failure. Voriconazole via nasogastric tube was unsuccessful because of large residual volumes in the stomach. Patient died on day 30 post-admission. Autopsy showed a necrotizing right-sided pneumonia due to Aspergillus fumigatus
A positive result from a culture of a respiratory specimen or positive findings from a direct microscopic examination is present in only about half of patients with IA (Mennink-Kersten et al. 2004). The predictive value of a positive culture result depends largely on whether the patient is immunocompromised and ranges from 20 % to 80 % (Perfect et al. 2001). Given the ubiquitous nature of Aspergillus spores, differentiation of colonization from infection remains problematic. Two studies have examined the significance of isolation of Aspergillus species in ICU patients and have confirmed the poor positive predictive values (Tarrand et al. 2003). However, although culture and microscopic examination of respiratory tract samples are performed on a regular basis in most ICUs (once or twice weekly, as a means of surveillance), it is not an appropriate guide for clinical practice.
Serologic testing techniques based on the detection of circulating fungal cell wall components, such as galactomannan (GM) or β-d-glucan, and detection of circulating fungal DNA by PCR techniques hold promise for patients with hematologic malignancy, but they have not been systematically studied for the diagnosis of IA in the ICU. GM and β-d-glucan are polysaccharide fungal cell wall components that are released during tissue invasion and that can be detected in specimens of body fluids (e.g., serum and bronchoalveolar lavage fluid) obtained from patients with IA (Klont et al. 2004). Studies of neutropenic patients have revealed high rates of sensitivity (67–100 %) and specificity (86–99 %) (Pfeiffer et al. 2006; Maertens et al. 2002). However, in a retrospective observational study of a medical ICU population, serum GM was elevated in only 53 % of patients with IA (Meersseman et al. 2004). Detection of serum GM is probably not a sensitive marker for IA (especially in non-neutropenic patients), as demonstrated in lung and liver transplant recipients (Husain et al. 2004; Kwak et al. 2004). Viable fungi can endure in the lung tissue (with encapsulation by an inflammatory process), whereas circulating markers can remain undetectable because of clearance by circulating neutrophils. Bronchoalveolar lavage (BAL) fluid could be a better specimen for GM detection. In a prospective study performed at our unit, 110 patients (10 % of total population) underwent BAL for culture and GM detection, serum GM measurement, and CT scan. Only 22 % of the patients were neutropenic. Sensitivity and specificity of BAL GM detection with a cut-off value of 0.5 was 88 % and 87 %, respectively. This is in contrast with a sensitivity of only 42 % for serum GM (taken on the same day as BAL GM and using the same cut-off value) (Meersseman et al. 2008). The use of β- d -glucan detection in the ICU is hampered by false-positive results (associated with the use of albumin, wound gauze, hemodialysis, and bacterial infections) (Pazos et al. 2005; Digby et al. 2003; Ostrosky-Zeichner et al. 2005). GM detection yields fewer false-positive results, although the use of β-lactam antibiotics posed a problem in the past. Although some residual GM might still be present in piperacillin/tazobactam, the preparations seem no longer responsible for false-positive GM results (Mikulska et al. 2012). Plasma-lyte, a frequently uses resuscitation fluid, might also cause false positivity of the GM assay (Petraitiene et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


