Fig. 9.1.
Clinical presentation of oral C. albicans infections with erythematous patches and white pseudomembranes on the tongue of a patient with chronic mucocutaneous candidosis
C. albicans interacts with epithelial cells via the processes of adherence, invasion and induction of cell damage (Naglik et al. 2011). Virulence factors are crucial in determining the role of opportunistic pathogens in infections. Important virulence factors expressed by C. albicans include dimorphism, the ability to grow in either yeast or filamentous form, adhesions factors, phenotypic switching, thigmotropism (ability to identify intercellular junctions at the mucosal surface by contact sensing and their targeted penetration) and secretion of hydrolytic enzymes such as lipase, phospholipase and proteinase (reviewed in Calderone and Fonzi 2001; Hube and Naglik 2001; Schaller et al. 2005). The interaction between virulence factors of C. albicans and host defence mechanisms plays a central role in determining whether colonization remains harmless or leads to infection of the epithelium and, possibly, systemic infection.
III. Epithelial Recognition and Signalling Detection Mechanisms
The innate immune system recognizes conserved pathogen associated molecular patterns (PAMPs), which represent broad groups of microbial species rather than a single specific species, through germline-encoded proteins, such as PRRs (Janeway and Medzhitov 2002). Recognition of C. albicans by the innate host defence system is mediated by PRRs of the TLR, CLR and nucleotide-binding domain, leucine-rich repeat (NLR) protein families (Franchi et al. 2010; Gauglitz et al. 2012; Naglik and Moyes 2011; Netea et al. 2008; Roeder et al. 2004; Weindl et al. 2010; Willment and Brown 2008). PRRs are expressed by various cells in the oral mucosa, including polymorphonuclear leukocytes (PMNs), dendritic cells (DCs), monocytes, macrophages, B cells, T cells and epithelial cells. Activation of PRRs leads to downstream signalling through pathways that induce transcription factors such as nuclear factor (NF)-κB, followed by enhanced transcription of antimicrobial peptides, cytokines, chemokines and co-stimulatory molecules. As such, PRRs are critical mediators between innate and adaptive immune responses. The relative contribution of specific PAMPs and their corresponding receptors in the oral mucosa remains to be elucidated in more detail.
A. Toll-Like Receptors in Epithelial Recognition of C. albicans
To date, most data regarding PRRs in the oral mucosa are available for TLRs. TLRs are a family of evolutionarily conserved receptors that react to bacterial, viral or fungal antigens or to endogenous factors released during cell injury. The capacity to recognize a variety of common microbial antigens and endogenous factors indicates that a primary function of TLRs is to act as sentinel receptors to alert the innate immune system to infection or tissue damage (Takeda et al. 2003; see also chapter “Receptor–Ligand Interactions in Fungal Infections” by Hardison and Brown).
To date, the TLR family comprises 10 members in humans (TLR1–TLR10) and 12 in the mouse (TLR1–TLR9 and TLR11–TLR13). All TLRs are characterized as type I transmembrane receptors with an extracellular leucine-rich repeat domain and a cytoplasmic tail with high similarity to the type 1 interleukin (IL)-1 receptor. The leucine-rich repeat domains of TLRs bind different microbial components (PAMPs), including bacteria cell wall molecules such as lipopolysaccharide and peptidoglycan, proteins (e.g. flagellin), as well as double-or single-stranded RNA of viruses or unmethylated CpG DNA. Ligation of TLRs leads to activation of a protease cascade, inducing transcription factors such as NF-kB and interferon regulatory factor (IRF)-3 and IRF-7 followed by enhanced transcription of antimicrobial peptides, cytokines, chemokines and co-stimulatory molecules. As such, TLRs function as critical mediators between innate and adaptive immune responses.
Several members of the TLR family are expressed constitutively in oral epithelial cells, healthy epithelial tissue (Beklen et al. 2008; Mahanonda and Pichyangkul 2007) and oral mucosa biopsies from patients with oral candidiasis (Ali et al. 2008). Previously, using a model of oral reconstituted human epithelium (RHE), we and others studied a number of different aspects of host–Candida interactions (de Boer et al. 2010; Moyes et al. 2010; Naglik et al. 2008; Schaller et al. 1998, 1999, 2002, 2004; Schaller and Weindl 2009; Wagener et al. 2012a; Weindl et al. 2007). Although the model consists of transformed cells (TR146; oral buccal carcinoma cell line) (Rupniak et al. 1985), all natural major markers of the epithelial basement membrane and of epithelial differentiation are expressed. More importantly, despite the artificiality of the model it behaves like human in vivo epithelium when treated with pathogens and pharmacologically active agents (Schaller and Weindl 2009) and it mimics the clinical setting of C. albicans infections in the oral cavity (Wilson et al. 2009). Analysis of the oral RHE by real-time RT-PCR demonstrated a high degree of similarity in TLR expression profiles between the oral RHE and buccal epithelial samples isolated from healthy individuals (Weindl et al. 2007). In the oral RHE model, all TLR gene transcripts except TLR7 at a low level are constitutively expressed. Similarly, in samples from healthy individuals, all TLRs except TLR5 and TLR7 are detected. The most commonly expressed TLR genes in vivo appear to be those encoding TLR1, TLR2, TLR4 and TLR8, with TLR1 being the most highly expressed gene.
Increased expression of TLR2 and TLR4 has previously been observed in inflamed gingival epithelial tissues (Sugawara et al. 2006). The immunohistochemical expression of nine TLRs (TLR1 to TLR9) was demonstrated in a series of sections from chronic hyperplastic candidiasis, leukoplakia and healthy tissue (Ali et al. 2008). Although oral epithelial cells express TLRs, no studies have yet demonstrated TLR upregulation upon stimulation with C. albicans. Heat-killed C. albicans cells failed to modulate epithelial TLR expression (Pivarcsi et al. 2003). Similarly, in our infection model of oral candidiasis, both heat-killed and viable C. albicans cells were unable to upregulate epithelial TLR expression despite the fungus causing clear signs of mucosal damage (Weindl et al. 2007). With regard to commensal organisms, it has been suggested that rapid responsiveness by epithelial TLRs may be detrimental to the host by causing an immune overreaction (Strober 2004). Thus, one possible explanation for the lack of direct TLR upregulation by C. albicans could be because the fungus is usually a harmless colonizer of oral mucosal surfaces in approximately 40% of healthy individuals (Arendorf and Walker 1979) and may even actively downregulate epithelial responses by unknown mechanisms (our unpublished data). In addition, during the carrier state, it would serve little purpose for the host to activate a TLR-mediated inflammatory response when it is not required.
B. C-Type Lectin Receptors in Epithelial Recognition of C. albicans
In contrast to TLRs, much less is known about the expression of CLRs in the oral cavity. The CLRs are a large superfamily of proteins characterized by C-type lectin-like domains (Zelensky and Gready 2005). Importantly, these receptors mediate fungal binding, uptake and killing and also contribute to the initiation and/or modulation of the immune response to fungi (Hardison and Brown 2012; Netea et al. 2008; Willment and Brown 2008). The two most important receptors in epithelial recognition of C. albicans are the mannose receptor (MR) and dectin-1. For details on CLRs see chapter “Receptor–Ligand Interactions in Fungal Infections” by Hardison and Brown.
Mannose Receptor
The MR (CD206) is a prototypical type I (group VI) transmembrane protein and is mainly expressed by macrophages, as well as by DCs (Taylor et al. 2005b). After carbohydrate recognition, the receptor mediates internalization of pathogens by phagocytosis, induction of NF-κB activation and the production of numerous defensive cytokines (Gazi et al. 2011; Netea et al. 2006; Taylor et al. 2005a; van de Veerdonk et al. 2009b). More recently it has been shown, that the MR induces IL-17 production by Candida mannan in the absence of mitogenic stimulation even more potently than Gram-negative bacteria (van de Veerdonk et al. 2009b).
Dectin-1
Dectin-1 is a type II transmembrane receptor and belongs to the natural killer cell receptor-like CLRs (Brown 2006). The extracellular carbohydrate recognition domain (CRD) selectively binds β-glucan polymers, a major component of yeast and mycobacterial cell walls, and mediates the phagocytosis of zymosan particles and intact yeast (Herre et al. 2004; Underhill et al. 2005). Dectin-1 also synergizes with TLR2- and TLR4-induced signals inducing tumour necrosis factor alpha (TNFα), IL-10, transforming growth factor-β and maturation of DCs (Brown et al. 2003; Dillon et al. 2006; Gantner et al. 2003).
Currently, there are no data published on the role of mannose receptor (MR) in localized Candida infections. The receptor is expressed in keratinocytes (Szolnoky et al. 2001) and oral epithelial cells (Wagener et al. 2012b). However, gene expression analysis in the oral RHE model showed no significant differences upon infection with C. albicans (our unpublished data). In oral epithelial cells, MR blocking did not alter cytokine secretions levels of IL-6, IL-8 and GM-CSF upon stimulation with Candida cell wall components (Wagener et al. 2012b).
The function of dectin-1 in mucosal candidiasis has not been fully established, but several studies suggest that dectin-1 might play a crucial role in the mucosal immunity against Candida, at least in the intestine. Myeloid lineage cells in the intestinal tract express dectin-1, and the outgrowth of Candida in the digestive tract from dectin-1-deficient mice was disproportionally high, leading to occlusion and contributing to the increased mortality (Reid et al. 2004; Taylor et al. 2002, 2007). Furthermore, dectin-1 is paramount for IL-17 induction by Candida (Leibundgut-Landmann et al. 2007; Osorio et al. 2008). Patients with an impaired IL-17 production caused by STAT3 (signal transducer and activator of transcription 3) mutations (hyper-IgE syndrome) and CMC have recurrent Candida infections (Eyerich et al. 2008; Ma et al. 2008; Milner et al. 2008). Previous studies have failed to demonstrate dectin-1 expression in epithelial cells from the gastrointestinal tract (Rice et al. 2005), lung (Evans et al. 2005; Lee et al. 2009b) and gingiva (Laube et al. 2008). However, epidermal keratinocytes appear to express functional dectin-1 (Lee et al. 2009a) and its expression can also be induced by mycobacteria in airway epithelial cells (Lee et al. 2009b). We have demonstrated that dectin-1 is expressed in the oral RHE but gene expression is not inducible by C. albicans and dectin-1 ligands did not stimulate cytokine secretion (Moyes et al. 2010; Wagener et al. 2012b). This suggests that dectin-1 plays only a minor role in oral epithelial cell detection of C. albicans and that other PRRs might contribute to the interaction between the epithelial cells and C. albicans PAMPs.
As for dectin-2, DC-SIGN and Mincle, these receptors seem not to be expressed in oral epithelial cells (our unpublished data).
C. NOD-Like Receptors in Epithelial Recognition of C. albicans
Oral epithelial cells express members of the NOD-like receptor (NLR) family, NLRC1 (NOD1) and NLRC2 (NOD2), and stimulation with synthetic ligands strongly increased expression of antimicrobial molecules whereas proinflammatory cytokines were not induced (Sugawara et al. 2006; Uehara et al. 2005, 2007; Uehara and Takada 2008).
NLRs are a family of intracellular immune receptors characterized by leucine-rich repeats and a nucleotide-binding domain. Like TLRs, NLRs recognize microbial products, as well as other intracellular danger signals, thus triggering host defence pathways through the activation of the NF-kB response and inflammatory caspases (Martinon et al. 2009). Several members of the NLR family, including NLRP3 (also known as NALP3 and cryopyrin), form large multiprotein complexes, termed the inflammasome, which in turn activate caspase-1 leading to the processing and secretion of IL-1β and IL-18 (Bryant and Fitzgerald 2009). Recent reports link IL-1β production induced by C. albicans to the NLRP3 and NLRC4 inflammasome (Gross et al. 2009; Hise et al. 2009; Joly et al. 2009; Kumar et al. 2009; Tomalka et al. 2011; van de Veerdonk et al. 2009a, 2011). Of note, TLR2, dectin-1 and NLRP3 were shown to be crucial for protection against dissemination of Candida in a murine model of oral mucosal infection (Hise et al. 2009). However, at present it is not known to what extent oral epithelial cells contribute to the observed defence mechanism or how important these mechanisms are in local, mucosal anti-Candida responses.
Although C. albicans is not recognized by NLRC1 and NLRC2 (van der Graaf et al. 2006), two other NLR members, NLRP3 and NLRC4, might have an important function in the host defence against mucosal Candida infections (Hise et al. 2009; Tomalka et al. 2011). Interestingly, NLRP3 is strongly expressed by keratinocytes in non-keratinizing epithelia such as oral cavity and oesophagus (Kummer et al. 2007). The potential role of NLRP3 in oral epithelial cells is further supported by studies showing increased IL-1β and IL-18 levels upon stimulation with C. albicans (Mostefaoui et al. 2004; Rouabhia et al. 2002; Schaller et al. 2004; Tardif et al. 2004; Weindl et al. 2007). NLRC4 or NLRP3 deficiency results in strongly reduced pro-inflammatory and antimicrobial peptide responses in a murine model of oral C. albicans infection, but only NLRP3 plays an important role in preventing systemic dissemination (Tomalka et al. 2011).
D. Discrimination Between Pathogenic and Commensal State
Recent reviews have addressed how epithelial cells interact with Candida and discussed the epithelial detection mechanisms that might enable mucosal tissues to discriminate between the “pathogenic” or “commensal” state of C. albicans (Moyes and Naglik 2011; Naglik et al. 2011). Briefly, C. albicans recognition activates two main epithelial signalling pathways, the MAPK and NF-κB pathways (Moyes et al. 2010). In oral epithelial cells, C. albicans hyphae (but not yeast) specifically activated the MAPK-p38 signalling pathway, which results in c-Fos transcription factor activation and the induction of proinflammatory cytokines. Importantly, this hypha-mediated response is highly dependent on fungal burdens. This indicates that MAPK-p38/c-Fos activation may constitute a “danger response” mechanism that is kept in check to permit immune quiescence in the presence of low C. albicans burdens but immune activation when C. albicans burdens increase and become hyphal. The MAPK–p38/c-Fos pathway is only activated by hypha-forming Candida species (C. albicans and C. dubliniensis, but not C. tropicalis, C. glabrata, C. parapsilosis or C. krusei) (Moyes et al. 2012) and is also present in human vaginal epithelial cells (Moyes et al. 2011). Therefore, this MAPK–p38/c-Fos pathway may represent a mechanism enabling different epithelial tissues to “recognise” dangerous Candida hyphae, thereby potentially identifying when this commensal fungus has become pathogenic. In is not yet known how hyphae activate this MAPK–p38/c-Fos response mechanism but it appears to be independent of cell wall polysaccharides including β-glucan, chitin and mannan (Murciano et al. 2011).
IV. Immune Responses Induced by C. albicans at Mucosal Surfaces
A. Modulation of Innate Immune Responses
During the period of colonization, extensive fungal growth is limited through release of antimicrobial peptides from epithelial cells or due to existence of other bacteria of the microbial flora. In this stage of colonization without clinical symptoms and signs of inflammation, neither C. albicans nor the host induce an inflammatory cytokine response. However, when these homeostatic conditions are disturbed, for instance due to antibiotic therapy or immunosuppression, superficial or even systemic infections may occur.
Oral candidiasis is a common opportunistic infection associated with HIV infection and may occur in 50% of untreated HIV-positive subjects and 90% of AIDS patients (Challacombe and Naglik 2006). Thus, it is tempting to speculate whether TLRs play a role in the development of opportunistic infections during HIV infection. In fact, in HIV-infected patients the risk of developing active tuberculosis (Ferwerda et al. 2007) and the occurrence of serious co-infections (Papadopoulos et al. 2010) positively correlates with the presence of common TLR4 polymorphisms. In contrast, polymorphisms of TLR2 or TLR4 do not influence susceptibility to oropharyngeal candidiasis in HIV-infected patients (Plantinga et al. 2010). However, functional studies were performed on leukocytes with heat-killed C. albicans, thus the relative contribution of TLRs on oral epithelial cells remains unclear.
1. Epithelial Cytokine Responses
During oral infection with Candida species, particularly with C. albicans, oral epithelial cells secrete a large number of cytokines, which maintain a central role in the protection against fungal organisms (Dongari-Bagtzoglou and Fidel 2005; Schaller et al. 2002). The cytokines are involved in enhancement of proliferation, activation and fungicidal activity of immune cells. In general, proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, TNFα, GM-CSF and others) regulate leukocyte trafficking (Eversole et al. 1997) and/or activate a strong antifungal response by oral epithelial cells (Schaller et al. 2002, 2004; Weindl et al. 2007). Our data support the hypothesis that C. albicans infection induces an epithelial cytokine pattern that may favour a chemotactic and Th1-type immune response and an environmental switch from an anti- to a proinflammatory milieu. However, epithelial cytokine production induced by C. albicans has not yet been associated with specific PRRs. C. albicans strongly induce GM-CSF and IL-8 in human oral epithelial cells and in three-dimensional models (Dongari-Bagtzoglou and Kashleva 2003; Weindl et al. 2007). Recent data indicate that TLR4 is not involved in Candida-induced GM-CSF and IL-8 production in epithelial cells (Li and Dongari-Bagtzoglou 2009; Weindl et al. 2007). The adhesion receptor CDw17 (lactosylceramide) might be responsible, at least partially, for GM-CSF activation mediated by NF-kB (Li and Dongari-Bagtzoglou 2009). Further studies are necessary to identify the receptors that mediate the epithelial cytokine response to C. albicans.
In response to infection and inflammatory stimuli, epithelial cells are also capable of inducing antimicrobial peptides such as defensins, cathelicidins and histatins (Diamond et al. 2008), which control C. albicans growth and infection. Among these peptides, human β-defensin-2 (hBD-2), hBD-3 and cathelicidin (LL-37) exhibit potent anti-candidal properties (Schneider et al. 2005). In this regard, we observed that the regulation of expression of the antimicrobial peptides hBD-1, hBD-2 and hBD-3 in the oral RHE also correlated with the degree of tissue damage caused by C. albicans (our unpublished results). These observations support the hypothesis of an active host-fungus interaction at the epithelial surface and provide insight into the molecular events leading to recruitment and activation of immune cells by the oral epithelium.
Recently, specific human gene mutations and polymorphisms have been linked to signal pathways resulting in susceptibility to C. albicans . STAT3 mutations identified in patients with hyper-IgE syndrome have been attributed to a defective IL-17 production and a diminished Th17 response, resulting in recurrent mucosal Candida infections (Eyerich et al. 2008; Ma et al. 2008; Milner et al. 2008). Similarly, deficiency in dectin-1 signalling pathways have been linked to CMC (Ferwerda et al. 2009; Glocker et al. 2009). Both studies, however, need to be interpreted with caution because both patient groups showed mucocutaneous manifestations, whereas the functional studies were performed on leukocytes. It remains to be proven whether the key mechanisms in these cases of severe candidiasis consist of impaired dectin-1 signalling at the epithelial level or impaired leukocyte activation of epithelium, mediated through cytokines such as IL-17 and IL-22. In addition to STAT3, dectin-1, IL-17RA and IL-17F polymorphisms, other polymorphisms affecting STAT1, DOCK8, TYK2, CARD9 and AIRE also appear to predispose patients to Candida infections (Plantinga et al. 2012).
2. Interaction with Immune Cells
Polymorphonuclear leukocytes (PMNs) are a central component of the innate immune response. In many mucosal infections and inflammatory disorders, the combination of epithelial injury, disease activity and symptoms parallel PMN infiltration of the mucosa. Similarly, during oral Candida infections, transepithelial migration of PMNs is believed to play a crucial role in the clearance of infection and in epithelial homeostasis (Fidel 2002). During infection, local production of multiple cytokines and chemokines induces the recruitment and activation of PMNs from the vascular compartment to the oral mucosa. IL-8 and GM-CSF are key chemokines involved in the recruitment of PMNs to sites of infection (Godaly et al. 2001; Reaves et al. 2005) and are rapidly activated by exposure to proinflammatory cytokines including IL-1α, IL-1β, IL-6 and TNFα (Yamashiro et al. 2001). The oral epithelium produces high levels of IL-8 and GM-CSF in response to C. albicans infection (Schaller et al. 2002, 2004; Weindl et al. 2007), which might explain the substantial migration of PMNs into Candida-infected oral mucosal sites. Addition of PMNs to our in vitro model of oral candidiasis enhanced a Th1-type immune response [interferon (IFN)-γ, TNFα], downregulated the expression of the Th2-type cytokine IL-10 and was associated with protection against Candida-induced tissue damage (Schaller et al. 2004). PMNs could protect the epithelium from C. albicans-induced cell injury via a process that was independent of phagocytosis, PMN transmigration or physical PMN–epithelial cell contact. Interestingly, the immunological crosstalk between C. albicans-infected oral epithelium and PMNs causes PMN-mediated upregulation of epithelial TLR4 (Weindl et al. 2007). Furthermore, epithelial TLR4 is directly responsible for protecting the mucosal surface from fungal invasion and cell injury. Noteworthy, cytokines such as IL-1α, IL-1β, IL-6, IL-8, macrophage inflammatory protein (MIP)-1β, monocyte chemoattractant protein (MCP)-1 and GM-CSF seem not to have an essential function in direct host defence against invading fungi, even in the presence of PMNs. Incubation of the RHE with neutralizing antibodies had a minimal effect on epithelial cell damage induced by C. albicans, and strong TLR4 upregulation was still observed after PMN addition. In contrast, incubation of the supplemented PMNs with all neutralizing antibodies, except GM-CSF, led to strongly reduced TLR4 mRNA expression. Thus, cytokines are crucial for the activation of PMNs and/or are released from the PMNs, which in turn results in upregulation of epithelial TLR4 and protection from fungal invasion (Fig. 9.2). Among the cytokines, TNFα showed the most potent effect, which confirms the important role of this cytokine in host defence against opportunistic fungal infections (Filler et al. 2005). Absence of this cytokine strongly impairs neutrophil recruitment and effective phagocytosis of C. albicans (Netea et al. 1999).
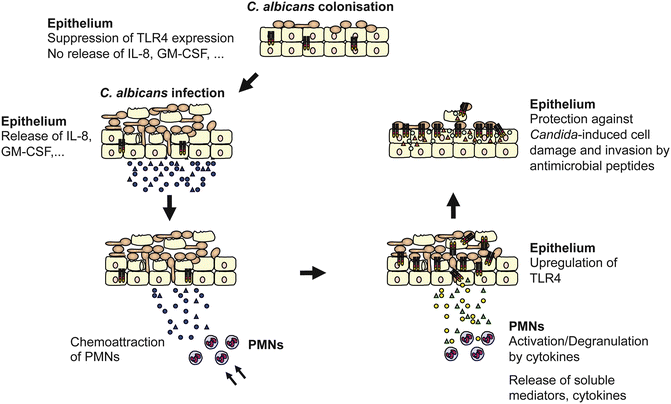

Fig. 9.2.
Model of TLR4-mediated and PMN-dependent antifungal defence by the oral epithelium. Epithelial cells control fungal cell growth and invasion. During colonization of the oral epithelium, C. albicans suppresses TLR4 expression and does not induce cytokine production. Infection, particularly in immunocompromised patients, leads to increased cytokine secretion that recruits and stimulates PMNs at the site of infection. After recruitment, several cytokines, especially TNFα, are directly involved in initiating the subsequent PMN-mediated upregulation of epithelial TLR4 via a process that does not require PMN infiltration of the mucosal tissues. Finally, epithelial TLR4 directly protects the oral mucosa from fungal invasion and cell injury by production of antimicrobial peptides. Circles and triangles represent secreted cytokines, antimicrobial peptides and other soluble mediators by the oral epithelium or PMNs, respectively. TLR4 expression is depicted in the oral epithelium (Modified with permission from Weindl et al. 2010, 2011)
In an in vitro model of oesophageal candidiasis, co-incubation of PMNs with C. albicans strongly upregulated hBD-2 and hBD-3 in oesophageal cells compared to PMNs or C. albicans alone (Steubesand et al. 2009). We recently observed that the addition of PMNs to the Candida-infected oral RHE model not only strongly upregulates epithelial TLR4 expression, but also promotes the release of LL-37, which correlated directly with protection (our unpublished data). The protective effect of LL-37 was abolished by the addition of TLR4-specific neutralizing antibodies and by TLR4 knockdown by RNA interference (RNAi), demonstrating the direct role of LL-37 in the protective process. We confirmed the protective role of LL-37 by exogenous addition, which reduced C. albicans-induced cell damage in the absence of PMNs. It is known that membrane permeabilization of C. albicans drives the antimicrobial activity of cathelicidins such as LL-37, although the precise mechanisms for Candida cell killing have not yet been identified (den Hertog et al. 2005; López-García et al. 2005). In addition, LL-37 also modulates TLR-induced cytokine responses in epithelial cells (Filewod et al. 2009). The detailed role of LL-37 in the protection from Candida-induced cell damage is currently under investigation. In summary, increased PMN-dependent production of antimicrobial peptides by epithelial cells could contribute to the protective effect and further underlines the important role for PMNs in clearance of experimental oral candidiasis.
B. Mucosal T Cell Responses
Activation of the innate immune system by C. albicans induces the secretion of a variety of proinflammatory cytokines and the expression of co-stimulatory molecules. It is generally accepted that induction of a Th1-type cellular response is crucial for the defence against C. albicans (Fidel et al. 1997; Romani 1999; Schaller et al. 2004). In contrast, a Th2 cellular response is considered non-protective because it induces a class-switch to non-opsonizing antibody subclasses and IgE (Clemons and Stevens 2001; Savolainen et al. 1996). Investigation of the role of Th17 in mediating the immune response has shown that Th17 memory cells are induced by Candida hyphae (Acosta-Rodriguez et al. 2007; Zhou et al. 2008). In a murine model, IL-17AR knockout mice had an increased susceptibility to systemic (Huang et al. 2004) and oropharyngeal candidiasis (OPC) (Conti et al. 2009). On the other hand, deleterious effects of IL-17 inflammatory activities have also been demonstrated (Bozza et al. 2008; De Luca et al. 2007; Zelante et al. 2007). Patients with an impaired IL-17 production suffer from mucosal C. albicans infections in hyper-IgE syndrome and CMC (Eyerich et al. 2008; Ma et al. 2008; Milner et al. 2008). Furthermore, in cases of autoimmunity with neutralizing antibodies to Th17 cytokines (IL-17A, IL-17F and IL-22), there is an increased incidence of CMC (Kisand et al. 2010). In contrast to Th cells, regulatory T (Treg) cells suppress inflammatory responses in disseminated C. albicans, resulting in higher susceptibility in mice (Netea et al. 2004; Sutmuller et al. 2006). However, the tolerization-inducing effects of Treg cells seem to be beneficial at mucosal sites (De Luca et al. 2007; Vignali et al. 2008). Treg cells may also promote Th17 cells in vitro and enhance host resistance in experimental oral candidiasis in mice (Pandiyan et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


