Intubation and Upper Airway Management
The first known use of positive-pressure ventilation (PPV) as a medical intervention dates back to the 16th century, as described in Vesalius’ de Humani Corporis Fabrica:
“But that life may in a manner of speaking be restored to the animal, an opening must be attempted in the trunk of the trachea, into which a tube of reed or cane should be put; you will then blow into this, so that the lung may rise again and the animal take in air. Indeed with the slight breath in the case of the living animal, the lung will swell to the full extent of the thoracic cavity, and the heart become strong.… for when the lung, long flaccid, has collapsed, the beat of the heart and arteries appears wavy, creepy, twisting; but when the lung is inflated at intervals, the motion of the heart and arteries does not stop.”1
Vesalius apparently resuscitated a Spanish nobleman by inflating his lungs through the trachea, resulting in resumption of cardiac activity and nearly in Vesalius’ death at the hands of the inquisitors. Vesalius, an excellent anatomist who disproved many of the cherished teachings of Galen that had been accepted as absolute truth for 13 centuries, was viewed as a heretic by his peers. In fact, one described him as “an impious madman who is poisoning all of Europe with his vaporings.”
Because of the lack of enthusiasm in response to Vesalius’ findings and writings, a 100-year hiatus attended the next attempt at endotracheal ventilation. In 1667, Robert Hooke, a prominent mathematician, geologist, and paleontologist kept a dog alive by intermittently insufflating air into its trachea using a set of bellows. One century later, in 1744, John Fothergill, one of the founders of the British Humane Society, described successful mouth-to-mouth resuscitation.
Because of concerns over development of emphysema and tension pneumothorax as complications of PPV (which was recognized as early as 1827), research on artificial ventilation in the nineteenth and early twentieth centuries focused primarily on negative-pressure ventilation (NPV). Iron lungs, tank ventilators, cuirass ventilators, and a variety of strange and remarkable differential pressure chambers and boxes were developed in the United States and Europe. The devices were powered by hand, water, steam, or electricity, and, in some cases, by the patient himself. However, PPV became incorporated into the resuscitation strategy of the Dutch Humane Society, which advocated mouth-to-mouth ventilation in conjunction with external thoracic and abdominal compression. In 1776, John Hunter described an apparatus that blew fresh air into the lungs with one set of bellows and sucked “bad” air out with a second set.
By the end of the nineteenth century, a surge in the evolution of thoracic surgery led to the use of tracheal intubation and PPV through cuffed tubes as acceptable components of medical care. An American surgeon, Joseph O’Dwyer, designed a series of metal tubes that were inserted between the vocal cords of children afflicted with diphtheria. Rudolph Matas referred to O’Dwyer’s devices in describing “intralaryngeal intubation” and “insufflation” and noted that, “the procedure that promises the most benefit in preventing pulmonary collapse in operations is … the rhythmic maintenance of artificial respiration by a tube in the glottis.” In the early part of the twentieth century, Franz Kuhn, a German surgeon, described techniques for oral and nasal intubation using flexible metal tubes introduced into the trachea with the assistance of the operator’s index finger; the procedure was preceded by application of topical anesthesia using cocaine. The airway was then sealed with a supralaryngeal flange and gauze packing.
Among the further advances that followed was the first laryngoscope created by Alfred Kirstein in Berlin. However, his model was never widely accepted. Chevalier Jackson developed a U-shaped laryngoscope that otorhinolaryngologists still use for endoscopy but was never adopted by anesthesiologists. In 1913, Janeway described an endotracheal tube with a removable cuff, an anesthesia ventilator, and a battery-powered laryngoscope. From 1900 to 1920, Dorrance, Elsberg, Löwen, and Sievers published methods for tracheal intubation and PPV.
The most influential figure in the history of endotracheal intubation is Sir Ivan Magill. Along with Stanley Rowbotham, he used anesthetics on Royal Army casualties during World War I (in particular, on patients with disfiguring facial injuries). Their patients were often intubated nasally to allow freer access to the face by the surgeon. Magill’s inventions include the Magill forceps, which is still used to facilitate nasal intubation, semirigid endotracheal tubes fashioned from mineralized rubber, and the Magill circuit, an L-shaped laryngoscope. He is also credited with describing the “sniffing position.”
Arthur Guedel, an American contemporary of Magill, refined the cuffed endotracheal tube and, by extensive experimentation on animal tracheas, determined that the best position for the cuff was just below the vocal cords. He popularized use of the cuffed endotracheal tube by publicly anesthetizing his pet dog, “Airway,” and immersing the animal in a tank of water. Upon awakening, the dog shook itself off and departed the arena.2
In current practice, access to the trachea through the nasopharynx or oropharynx takes advantage of laryngoscope blades invented in the 1940s by Robert Miller, a Texas clinician, and Robert Macintosh, an Oxford professor. The Miller blade was an advance over similar straight blades; it was designed to pick up the epiglottis and expose the vocal cords. The curved Macintosh blade differed from previous models and was designed for insertion between the epiglottis and tongue. Although many variants of the two blades are available today, including those with different angulations, prisms, and fiberoptic bundles, the Miller and the Macintosh blades remain the mainstays of the anesthesiologist’s armamentarium.
The first departments of anesthesiology were founded in the early 1940s. Thereafter, the skills required for management of the upper airway and endotracheal intubation became widely disseminated in the United States and the United Kingdom.
UPPER AIRWAY ANATOMY AND CLINICAL RELEVANCE
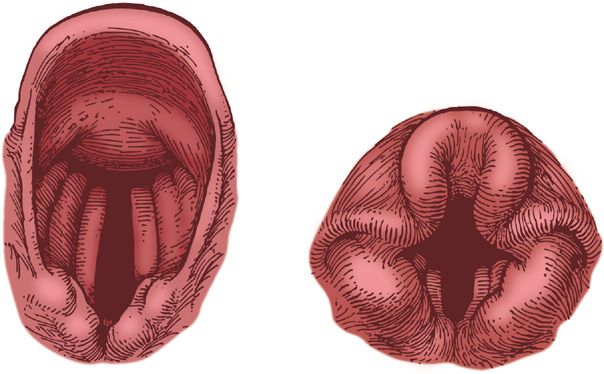
The two functional conduits between the trachea and atmosphere—the oropharynx and the nasopharynx (Fig. 146-1)—join at the level of the base of the skull to form the hypopharynx. The oropharynx includes the base of the tongue, uvula, and tonsils. The nasopharynx is separated from the oropharynx by the mobile soft palate. The hypopharynx includes the vallecula, which is the space posterior to the tongue and anterior to the cervical esophageal inlet. Typically, the adult epiglottis is crescentic, moderately stiff, and thin. Because of its ligamentous attachments, the adult epiglottis can be lifted indirectly using a curved laryngoscope blade applied to the base of the tongue. The U-shaped infant epiglottis is longer and floppier than the epiglottis of the adult. Therefore, a straight blade is typically required to lift the infant epiglottis directly during endotracheal intubation.
Figure 146-1 Comparative anatomy of adult and infant airways. (Reproduced with permission from Barash P, et al, eds. Clinical Anesthesia. Philadelphia, PA: Lippincott; 1989:544, D. Factor, illustrator.)
The adult and infant airways differ in several other respects. The narrowest portion of the adult airway is the rima glottidis, the area between the vocal cords; in contradistinction, the cricoid is the narrowest portion of the infant’s airway. The infant larynx is also situated relatively more cephalad than the larynx of the adult; in addition, the vocal cords of the infant are angled, whereas the vocal cords of the adult are perpendicular to the airway.
In an awake patient, with the head in the neutral position (i.e., neither flexed nor extended), air moves freely through both the oropharynx and nasopharynx. In most normal subjects, the same is true during sleep. Abnormalities of any of the component parts of the upper airway can impede airflow during respiration while awake; alternatively, impeded airflow may only become evident during sleep (e.g., as snoring or obstructive apnea). Consequently, a directed history and physical examination should be performed prior to any procedure on the airway.
A history of nasal polyps or nasal septal deviation mandates caution prior to nasotracheal intubation, transnasal passage of a fiberoptic scope, or insertion of a nasal airway. The patient’s sleeping partner is often the best source of information about snoring and apnea, manifestations that may result from a variety of upper airway abnormalities, including soft-tissue redundancy, masses, polyps, stenosis, or lymphoid hypertrophy from the nose to the hypopharynx and larynx. Vocal changes or abnormalities may suggest abnormalities of the vocal cords and warrant preintubation evaluation.
The physical examination of the airway is preceded by a conversation with the patient. Hoarseness, stridor, tachypnea, and coughing suggest potential upper airway problems. The examination then can be pursued systematically beginning with the nasopharynx. The patient’s ability to breathe through a single nostril (when the mouth is closed and the other nostril occluded) indicates that the passage is relatively patent. Asymmetry often exists between the two sides and, whenever possible, instrumentation should be performed on the more patent side.
The ability to open the mouth is limited in patients with temporomandibular joint disease. The temporalis muscle may be scarred or fibrotic (e.g., secondary to prior radiation) resulting in restricted mandibular mobility. Fractures to the mandible produce limited ability to open the mouth that, when the limitation is caused by muscle spasm, disappears with anesthesia. Some fractures functionally affect the mobility of the jaw, irrespective of anesthetic state. Inability to open the mouth more than 40 mm is considered to be clinically significant.
The patient’s dentition should also be assessed prior to elective management of the airway. Protruding maxillary incisors (“buck teeth”) interfere with direct laryngoscopy by restricting the extent to which the laryngoscope blade can be aligned with the trachea. Dental caps and other prostheses are fragile and easily damaged during laryngoscopy. The laryngoscope may become lodged in gaps between the maxillary teeth during instrumentation and interfere with intubation. Severe dental caries or periodontal diseases make it easier to dislodge teeth during airway instrumentation. The edentulous patient often has an atrophic mandible and large tongue and may be difficult to ventilate by mask because of poor fit of the mask. Intubation of the trachea in such a patient becomes difficult because the tongue, no longer constrained by the teeth, interferes with visualization of the larynx.
Abnormalities of the tongue, hard palate, tonsillar pillars, and hypopharyngeal structures can impede or prevent intubation. Normally the tongue is small and sufficiently flexible to be displaced by a laryngoscope blade during visualization of the vocal cords. However, the tongue is enlarged in obese patients, those with angioedema or impaired lymphatic drainage (e.g., after cervical surgical procedures or trauma), or in the setting of certain neoplasms. Burns, scars, or radiation of the submandibular soft tissue prevent lateral displacement of the tongue into the oropharynx during laryngoscopy. Similarly, in patients with small jaws (“receding chins”), displacement or flattening of the tongue during laryngoscopy is difficult, making intubation a challenge. A hyomental distance (the distance from the hyoid bone to tip of the mandible) of less than 6 cm should raise awareness of potential difficulty with intubation.
A cleft or high, arched palate is seen in a variety of congenital abnormalities of the facial bones, including the Treacher Collins, Pierre Robin, Klippel–Feil, Goldenhar, Beckwith–Wiedemann, and Crouzon syndromes, as well as the mucopolysaccharidosis. Affected patients are difficult or impossible to intubate using standard approaches.3
Intraoral, oropharyngeal, hypopharyngeal, and laryngeal lesions, as well as tonsillar hypertrophy, can interfere with both laryngoscopy and ventilation by mask. The epiglottis can be infiltrated, inflamed, floppy, or enlarged by fat. The retropharyngeal and lateral pharyngeal spaces are continuous and therefore subject to expansion by processes that involve the mediastinum (e.g., the presence of edema, blood, pus, or soft-tissue emphysema). Patients with epiglottitis and parapharyngeal swelling often exhibit a characteristic posture, sitting upright in the sniffing position and drooling.
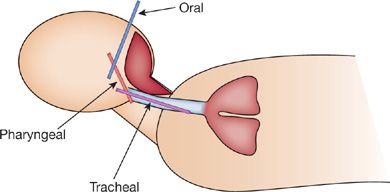
The preferred position for visualization of the vocal cords is the sniffing position (Fig. 146-2). However, this position may be unsuitable in some patients or impossible to achieve in others.
Figure 146-2 The sniffing position with the oral, pharyngeal, and tracheal axes.
The normal range for flexion and extension of the neck ranges from 90 to 165 degrees. A variety of disorders limit this range. Patients with cervical osteophytes or ankylosing spondylitis, who are often fixed in an anteroflexed head position, may be difficult to intubate. Halo fixation imposes similar constraints. Rheumatoid arthritis, which may affect the cervical spine even in asymptomatic patients, may be problematic. By the age of 75 years, the normal aging process results in as much as a 20% reduction in cervical spine mobility. Injury to the cervical spine or the presence of a cervical collar also impairs the ability of the laryngoscopist to position the head. Finally, patients with short, muscular necks have limited neck mobility and redundant soft tissue in the mouth and submandibular space, making airway visualization a challenge.
A variety of other anatomic features, including large breasts or a barrel chest, can complicate airway management by interfering with the excursion of the butt of the laryngoscope blade. During pregnancy, the oral and pharyngeal mucosae are swollen and bleed easily. When associated with a diminished functional residual capacity and increased volume of acidic gastric contents, intubation becomes quite hazardous.
The epidemic of obesity in Western societies has created a new population of patients with airway “abnormalities.” Patients with a body mass index greater than 30 may be at increased risk for obstructive sleep apnea and gastroesophageal reflux. On physical examination, these patients often have some combination of macroglossia, a narrower, bulkier oropharynx, decreased neck mobility—all of which can complicate airway management. Some patients have a cervical fat pad, which may prevent optimal head positioning during intubation.4
Based upon anatomical considerations, clinicians commonly employ the Mallampati scale (Table 146-1)5 to evaluate objectively the airway’s suitability for placement of the endotracheal tube. The ability to visualize the soft palate, fauces, tonsillar pillars, and uvula is used to predict the degree of difficulty in exposing the larynx. A careful examination of the airway, coupled with attention to difficulties during prior procedures and the physical features described above, permit adequate preparation for instrumentation of the difficult airway.
UPPER AIRWAY MANAGEMENT
Airway management is well suited to the use of algorithms. The American Society of Anesthesiologists has published a “difficult airway” algorithm for use in the operating room.6 In addition, algorithms for the critical care unit7 and trauma setting8 have also been developed. In using an algorithm-based approach, the first decision branch point typically addresses the need for endotracheal intubation, since short-term respiratory insufficiency often can be managed noninvasively.
Factors that must be considered in the care of patients with respiratory compromise include the level of consciousness, clinical context (e.g., the perioperative setting, emergency circumstances, etc.), anticipated duration of respiratory problem, risk of gastric aspiration, airway patency, concurrent medical problems, and anticipated relative ease of noninvasive (i.e., spontaneous or mask ventilation) versus invasive (i.e., endotracheal intubation) management of the airway.
In the patient with neurological depression due to injury of the central nervous system, noninvasive management is usually inappropriate due to the potential for developing hypercarbia or hypoxia and exacerbation of the primary injury. Conversely, in the patient sedated or obtunded by drugs or seizures, the clinical state is often brief in duration, so temporizing measures may be appropriate.
Several factors differentiate elective perioperative airway management from emergency care. During surgery, an anesthesiologist or anesthetist is constantly present; the patient is properly prepared (i.e., the stomach is empty and a drying agent has been administered) and the environment is designed to facilitate airway management (i.e., there is ready access to suction, a ventilator, etc.). Under these circumstances, caregivers may choose to sedate the patient to the point of semi-obtundation. Conversely, in an emergency, the setting is usually less than optimal. Airway management is usually only one component of the care rendered during cardiac or trauma resuscitation, and definitive airway intervention is essential in order to allow care providers to concentrate on other problems.
Potentially quickly reversible processes (e.g., some attacks of asthma or episodes of pulmonary edema) may be appropriately managed without intubation. In other instances (e.g., blunt chest injury), the initial problem can be expected to worsen, and early intubation is warranted.
The volume and acidity of the patient’s gastric contents must be factored into any decision about management of the airway. Aspiration of solid food can be catastrophic, as can large volumes of acidic, enzymatically active gastric fluid. Most studies have indicated that aspirated stomach contents with a pH lower than 2.5 or volume greater than 0.5 to 1.0 cc/kg are likely to cause lung damage. The lung damage is manifested by loss of ciliated and nonciliated epithelial cells in the trachea, destruction of type I and II pneumocytes, depletion of surfactant, and increased vascular permeability (see Chapter 141). Pain and narcotics may alter gastric emptying or change gastric pH, as can a number of disease states, such as intestinal obstruction, diabetic gastroparesis, and obesity. Unless the patient has fasted for more than 8 hours and is not subject to the confounding factors noted above, a full stomach should be presumed, and airway management handled accordingly.
The patient’s coexisting medical problems and expected course must also be considered in management of the airway. For example, endotracheal intubation can be a dangerous stress to a patient with coronary artery disease and can be performed more safely after suitable preparation than under emergency circumstances. As another example, a patient with Fournier gangrene and normal lungs is appropriately managed by maintaining intubation and sedation between trips to the operating room for debridement, rather than by performing multiple extubations and reintubations. Similarly, elective intubation and mechanical ventilation can prevent aspiration or atelectasis in a patient with hepatic encephalopathy who is awaiting liver transplantation, thereby improving the likelihood of a successful outcome.
Some degree of airway obstruction can be managed without intubation by proper positioning of the head, use of an oral or nasal airway, or application of positive airway pressure (PAP) by mask. A rolled towel or small pillow placed behind the neck or occiput reproduces the sniffing position. Oral and nasal airways can alleviate airway obstruction due to redundant airway soft-tissue or muscle relaxation. The application of positive pressure to the mouth and nose (mask continuous positive airway pressure or mask CPAP) distends the soft tissue of the airway. For this reason, nasal mask CPAP is frequently used in the management of obstructive sleep apnea (see Chapter 99). These measures can be used as short-term, temporizing alternatives to intubation in the patient who is ventilating spontaneously in the intensive care unit or operative setting.
Although a growing literature exists on the use of noninvasive ventilation9–13 in a variety of settings that previously would have mandated endotracheal intubation, anesthesia is frequently administered in the operating room by mask using positive pressure. True mask ventilation is readily accomplished in the anatomically normal patient. However, some anatomic features, such as a beard, flat or sharp nose, or sunken cheeks (in the edentulous patient) can make mask-assisted ventilation difficult or impossible.
Indications for tracheal intubation (Table 146-2) fall broadly under several categories: respiratory failure, airway protection, hemodynamic instability, and perioperative management. If intubation is indicated, the clinician must decide upon the route and technique.