Introduction and Overview of Pacemaker/ICD Therapy in Children
Peter Aziz
J. William Gaynor
V. Ramesh Iyer
INTRODUCTION TO PACEMAKER THERAPY
HRS/American College of Cardiology Recommendations/Guidelines for Pacemaker Implantation
The clinical indications for pacemaker implantation in children, adolescents, and young adults with congenital heart disease (CHD) are summarized below.
Class I
Advanced second- or third-degree atrioventricular block (AVB) associated with symptomatic bradycardia, ventricular dysfunction, or low cardiac output.
Sinus node dysfunction (SND) with correlation of symptoms during age-inappropriate bradycardia.
Postoperative advanced second- or third-degree heart block that is not expected to resolve or that persists >7 days after cardiac surgery.
Congenital complete AVB with a wide ventricular escape rhythm, complex ventricular ectopy, or ventricular dysfunction.
Congenital complete AVB in an infant with an average rate of 55 bpm or less or a rate of 70 bpm or less in a patient with CHD.
Class IIa
CHD and sinus bradycardia for the prevention of recurrent episodic intra-atrial reentrant tachycardia (IART).
Congenital complete AVB beyond infancy with an average heart rate of <50 bpm, abrupt pauses in ventricular rate that are more than two to three times that of the sinus cycle length, or in patients with symptomatic bradycardia.
Sinus bradycardia in patients with complex CHD with a resting ventricular rate of <40 bpm or pauses of more than 3 seconds.
CHD and impaired hemodynamics due to sinus bradycardia or loss of atrioventricular (AV) synchrony.
Unexplained syncope in a patient with prior congenital heart surgery complicated by transient complete heart block with residual fascicular block.
Class IIb
Transient postoperative complete AVB that reverts to sinus rhythm with residual bifascicular block.
Congenital complete AVB in a patient with an acceptable rate, narrow QRS escape, and normal ventricular function.
Asymptomatic sinus bradycardia after biventricular repair of CHD with a resting heart rate <40 bpm or ventricular pauses of more than 3 seconds.
Class III
Not indicated for transient postoperative complete AVB.
Not indicated for asymptomatic bifascicular block after CHD surgery in the absence of prior transient AVB.
Not indicated for asymptomatic second-degree AVB type I.
Not indicated for asymptomatic sinus bradycardia with pauses <3 seconds and an average heart rate >40 bpm.
Common Clinical Indications for Pacemaker Implantation
Complete Congenital Heart Block
Complete congenital heart block (CCHB) is the most common indication for permanent pacemaker in the pediatric population in the absence of CHD. Approximately 20% to 30% of paced pediatric patients received implants for this indication. Though maternal exposure to lupus antibodies is a common etiology for CCHB, most cases are idiopathic. The importance of confirming lupus exposure is highlighted by the fact that some infants exposed to lupus antibody are also more likely to develop dilated cardiomyopathy as a result.
L-transposition, heterotaxy, and common AV valve defects are congenital lesions commonly associated with complete heart block. The most common association of CHB in the newborn is heterotaxy with left atrial isomerism. In these patients with significant fetal compromise (hydrops, poor ventricular function), outcomes remain poor. Isolated CCHB in the absence of CHD, however, carries an excellent prognosis.
Postoperative Heart Block
Before the advent of pacemakers, surgical complete heart block (SCHB) following repair of CHD carried a dismal prognosis with a 50% mortality rate. Currently, pacemaker implantation is recommended for persistent SCHB for at least 7 days, with no expected resolution. The study by Weindling et al. showed 97% recovery of AV node conduction by 10 days suggesting an extended observation period before making the decision of pacemaker implantation. Although the majority of patients with resolution of SCHB demonstrate intact AV conduction in follow-up, a small subset of patients develop recurrence of AV node disease several years after surgical intervention. As such, pacemaker implantation is a class II indication in patients with unexplained syncope and residual bifascicular block following resolution of SCHB as these patients may be at risk for recurrence or higher grade heart block. Unfortunately, no definitive predictors of recurrence have been established in patients with normal electrocardiograms (ECGs; after resolution of immediate postoperative heart block) and these patients require close follow-up attention postoperatively.
Epicardial vs. Transvenous Considerations
Epicardial pacing systems (Fig. 107.1) are generally utilized in younger patients (<5 years or so), patients with single ventricle physiology and intracardiac shunts. In a multicenter study by Khairy and colleagues comparing transvenous versus epicardial pacing systems, the authors found a greater than twofold increase in thromboembolic events with transvenous devices in patients with significant left-to-right or right-to-left shunts. The advantage of transvenous systems is that transvenous leads tend to have lower pacing thresholds, higher lead impedances, and longer battery longevity in comparison to the epicardial leads. Of course, epicardial systems also obviate the need for a sternotomy or thoracotomy. The risks of venous occlusion and lead compromise are significantly increased in infants with transvenous pacemakers because of smaller patient and vessel lumen size. The major disadvantage of epicardial systems in young children is the higher risk of lead fracture due to mechanical stress. More importantly, maintaining long-term vessel integrity is imperative for infants requiring life-long pacing and, therefore, an epicardial system is ideal at a young age until adequate vascular growth is achieved and transvenous leads are an option.
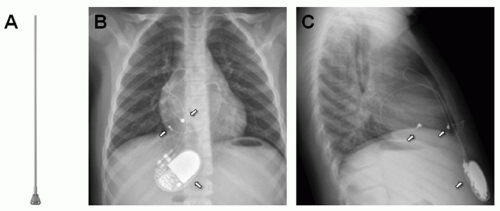
Transvenous leads in the pediatric patient pose a unique challenge. Adhesions between the leads and endocardial or vascular tissue and patient growth can cause shear and stress tension on the lead and may compromise lead function. To circumvent this issue of growth, many implanters leave an extra loop of lead (such as right atrial loops) to minimize tension (Fig. 107.2). However, looping may create the presence of more surface lead area in the heart and promote further adhesions, thus complicating future lead extraction if needed.
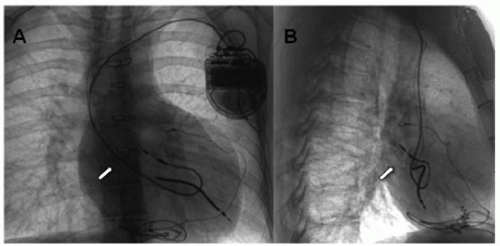
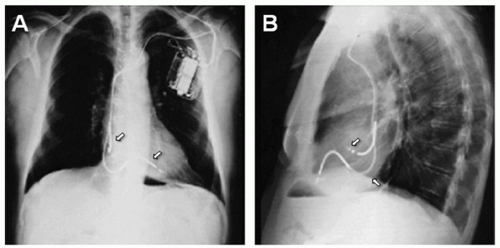 Fig. 107.2. X-rays showing transvenous atrial and ventricular lead (arrows pointing) with extra loop for growth in anteroposterior (AP) (A) and lateral (B) views. |
Though epicardial systems are preferred in infants, the decision regarding implantation type in young children is more difficult. The risk of thromobembolism in young patients must be balanced with the risk of compromise to lead integrity. In a study of 63 children, echocardiography and venography were routinely performed to assess the risk of thrombus formation. Twenty-one percent were found to have moderate or severe thrombosis at follow-up. Patients found to have thrombosis were younger (4.5 vs. 8.2 years) and had larger lead diameter to body surface area ratio suggesting that children <5 to 6 years of age should have epicardial systems implanted. Dual chamber (two lead) systems were also found to be prothrombotic (because of two leads occupying a larger vessel diameter) and should be avoided in the younger patient population.
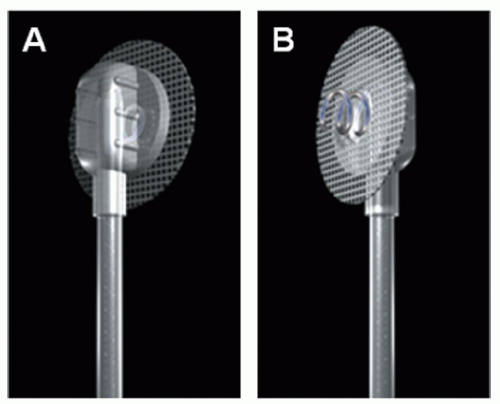
Epicardial systems (Fig. 107.1) are preferred in all patients with CHD and intracardiac shunting, regardless of age, because of increased risk of systemic thromboembolism. Unipolar leads (Fig. 107.1) have a single electrode with the pacemaker generator acting as the anode. Steroid-eluting leads provide lower (better) pacing thresholds and therefore preserve battery longevity. Bipolar leads (suture on) offer better sensing
thresholds as compared with unipolar leads since they avoid far-field cardiac or skeletal muscle sensing. Ideally, bipolar leads (Fig. 107.3) should be placed on the myocardium free of scar to optimize sensing and pacing thresholds. Bipolar screw-in leads are also available for use in older patients with thicker myocardium (Fig. 107.4). In patients with multiple prior sternotomies, the left thoracotomy approach enables the surgeon to access more posterior structures such as left atrium and ventricle and avoid scar tissue ( from sternotomies), thus facilitating dissection.
thresholds as compared with unipolar leads since they avoid far-field cardiac or skeletal muscle sensing. Ideally, bipolar leads (Fig. 107.3) should be placed on the myocardium free of scar to optimize sensing and pacing thresholds. Bipolar screw-in leads are also available for use in older patients with thicker myocardium (Fig. 107.4). In patients with multiple prior sternotomies, the left thoracotomy approach enables the surgeon to access more posterior structures such as left atrium and ventricle and avoid scar tissue ( from sternotomies), thus facilitating dissection.
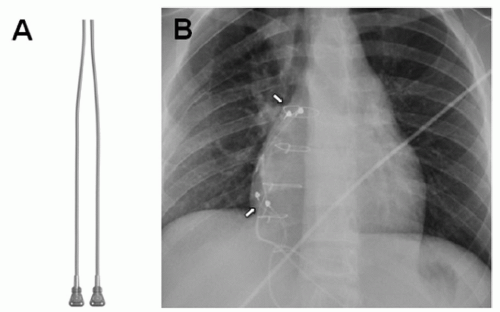 Fig. 107.3. Illustration of an epicardial bipolar suture on lead (A), X-rays of epicardial bipolar atrial and ventricular lead (arrows pointing) in an anteroposterior view (B). |
Subpectoral vs. Subcutaneous Considerations
Subpectoral devices are preferred in children as they offer the most optimal cosmetic result. However, the implant may be more technically challenging (more submuscular dissection) and need an experienced implanter. Muscular protection of the proximal lead, header, and generator improve longevity as well. Since subpectoral implants are anteriorly and posteriorly surrounded by muscular tissue, bipolar sensing and pacing are preferred to minimize muscular interference and muscular stimulation, respectively.
Pacing in Operated Congenital Heart Disease
The indications for pacemaker implantation in patients with and without CHD are important to distinguish. In patients with normal cardiac anatomy, bradycardia, even advanced atrioventricular heart block, can be well tolerated from a symptomatic standpoint and is not an indication alone for pacemaker implantation. However, patients with operated CHD may require closer evaluation for residual hemodynamic issues, which may influence pacemaker implantation decision-making. One such example is an adolescent with single ventricle physiology with AV valvar insufficiency who has SND with bradycardia and junctional rhythm. Such a subject may be a candidate for pacing (atrial pacing) to improve his symptoms and hemodynamics. Consideration must be given to residual hemodynamic issues such as AV valve or semilunar valve insufficiency and chamber enlargement in addition to the presence of sinus or AV node dysfunction.
Pacing for Arrhythmias
Intra-atrial Reentrant Tachyarrhythmias
Pacemaker implantation is recommended for patients with CHD and bradycardiainduced IART. The purpose of pacing in these individuals is to prevent premature atrial complexes and short-long-short extrastimuli sequences, which have been implicated as tachycardia triggers. Anti-tachycardia pacing is a useful programming tool available in select devices, which has been shown to terminate atrial reentrant tachycardia in more than 50% of instances in a multicenter trial of pediatric patients with CHD. The use of anti-tachycardia pacing is limited since it is not effective in terminating atrial fibrillation and may actually induce atrial fibrillation in susceptible patients.
Long QT Syndrome
Life-threatening arrhythmias such as polymorphic ventricular tachycardia (VT) (Torsade de pointes, TdP) can be triggered by bradycardia or pauses in some forms of long QT syndrome (LQTS). Cardiac pacing has been employed to prevent pause-dependent TdP and has been shown to be effective in preventing ventricular arrhythmias and sudden death. Physiologic pacing has also been shown to shorten the QTc interval. Pacing has also improved the clinical
outcome in LQTS patients with 2:1 AVB at birth, long to be considered extremely high risk for sudden cardiac death (SCD).
outcome in LQTS patients with 2:1 AVB at birth, long to be considered extremely high risk for sudden cardiac death (SCD).
Biventricular Pacing in Pediatrics
The objective of cardiac resynchronization therapy (CRT) is to coordinate right and left ventricular function in patients with clinical heart failure, particularly those with a left bundle branch block. Numerous studies in adult patients have demonstrated the benefits of CRT in those with dilated cardiomyopathy and electrome-chanical dyssynchrony. These controlled and randomized studies have shown an improvement in New York Heart Association (NYHA) class, exercise stress testing parameters, and mortality. Unfortunately, there are no such pediatric studies that have shown a similar benefit, but there are small series and case reports that have shown some promise of CRT in children with and without CHD. Currently, cardiac resynchronization is a class I indication in adult patients with an ejection fraction of ≤35%, QRS duration of ≥120 ms, and a NYHA class of III or IV.
The efficacy of CRT in the pediatric population, particularly those patients with CHD and right ventricular (RV) dysfunction, is not well established. As occurs in most studies in patients with CHD, the degree of heterogeneity in this population precludes large studies and conclusive statements. Moreover, traditional measures of improvement (QRS duration, tissue Doppler imaging (TDI), ventricular function) are complicated by underlying congenital cardiac lesions and establishing benefit in these patients is exceedingly difficult. In a small study, CRT was shown to increase arterial blood pressure and decrease QRS duration in the acute postoperative setting in patients with CHD. One prospective study by Jeewa et al. showed no acute benefit to CRT (cardiac index, blood pressure, TDI) in the congenital population. Long-term results in the largest retrospective study to date showed improved NYHA class and ejection fraction in 87% of patients undergoing CRT. Unfortunately, no conclusive study on CRT exists in pediatrics and most studies show conflicting results. It remains to be determined whether CRT will play a role in the management of patients with CHD, particularly those with RV dysfunction and right bundle branch block.
Surgical Considerations
Implantation of pediatric pacemakers is best performed by a team (cardiologists, surgeons, and anesthesiologists) skilled in the management of children. Particular appreciation for anatomical considerations is often the key in patients with CHD as anatomical variations often dictate the surgical approach. Ideally, general anesthesia should be performed by a pediatric cardiac anesthesiologist with special attention to effects of anesthesia on the hemodynamics of the cardiac lesion.
Careful screening for patients deemed pacemaker dependent (defined as an unreliable underlying escape rhythm) is a crucial part of surgical preparation. Anesthesia commonly suppresses conduction and may exacerbate brady arrhythmias. Pacemaker-dependent patients undergoing generator changes must also have an appropriate mechanism for maintaining hemodynamic stability. Isuprel administration or a temporary transvenous pacing lead via the femoral vein are useful adjuncts in preparation for pacemaker implantation in patients with unreliable escape rhythms.
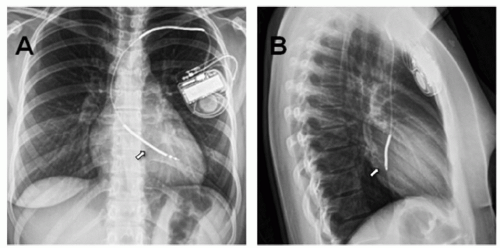
Patients with CHD frequently have variations in venous anatomy, thereby complicating the transvenous approach. As such, venography of the subclavian, innominate, and superior vena cava can document patency and provide a “road map” for venopuncture. Clear elucidation of intracardiac anatomy and the presence or absence of shunts is also imperative. The presence of an unrecognized patent foramen ovale may lead to inadvertent placement of the ventricular lead in the left ventricle (Fig. 107.5). This could be avoided by careful fluoroscopic imaging of the leads using different angles to confirm anterior lead placement. On the other hand, the ventricular lead placement in a patient with transposition of the great arteries (in the pulmonary ventricle-anatomically left ventricle) will be posterior fluoroscopically compared with a patient with normal segmental anatomy (Fig. 107.6). Demonstration of patent systemic venous return in patients with atrial switch operations (Mustard, Senning) is imperative as occlusion prevents transvenous access to endocardial tissue. Narrowing, stenosis, or interatrial leaks of the atrial baffle needs to be recognized by angiography and addressed (surgically, by stent or atrial septal defect device placement) before transvenous leads are placed (Figs. 107.6 and 107.7). Cardiac angiography in this patient population is also indicated to assess for baffle leaks that are a risk for paradoxical emboli and stroke. Baffle leaks need to be closed (either by device closure or surgical approach) before transvenous leads are inserted. The use of transvenous approach for atrial and ventricular pacing has been described in patients with single ventricle physiology and Fontan palliation. However, this poses technical challenge of finding viable atrial tissue capable of capture (in Fontan baffle with prosthetic material) and also obviates the need for chronic anticoagulation and its associated complications.
LEAD EXTRACTION
Summarized below are the indications for lead extraction as it pertains to the pediatric patient:
Class I
Sepsis related to device infection.
Life-threatening arrhythmia or immediate physical threat related to retained lead fragments.
Clinically significant thromboembolic event caused by lead fragment.
Lead that interferes with subsequent implantation.
Class II
Localized pocket infection.
Pocket site that causes significant discomfort.
Lead that poses potential threat due to lead design or failure.
Nonfunctional lead in a pediatric patient.
Class III
Risk of removal outweighs benefit.
Lead that can be reused at time of generator change.
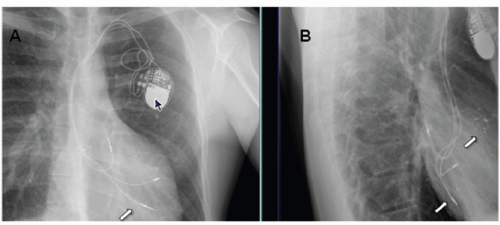
The most common class I indication for lead extraction is pocket/device infection (Fig. 107.8). Successful treatment of pacemakerrelated infection requires complete removal of all prosthetic (pacemaker and lead) material. In a study from the Cleveland Clinic of 123 patients undergoing hardware removal for infection, four patients experienced recurrence of infection and all four patients had retained device remnants, both transvenous and epicardial. Complete extraction was achieved in 118 patients, all of whom had no infection recurrence. Lead malfunction in the pediatric population is the most common indication for lead extraction. Lead insulation disruption and inner pacing or sensing coil fracture (Fig. 107.9) are common causes of lead malfunction, particularly in pediatrics as trauma and patient growth can compromise lead integrity. Traditionally, epicardial leads are abandoned (after new lead/s placement) without the need for extraction.
Thrombus formation around the lead is the major impediment to extraction. Fibrous tissue then forms over the lead in the course of days to months. Typical sites of fibrous accumulation (on the lead) include the venous entry site, the anastomosis of the superior vena cava and innominate vein, and the distal lead tip. In younger patients with older leads, calcification may further strengthen the fibrous matrix, thereby complicating extraction.
Several tools have been devised to facilitate lead extraction. In the past, direct traction was the only available method. Not surprisingly, direct traction often led to complete lead fracture with retained fragments requiring surgical removal. The advent of locking stylets allows for traction to be placed on the distal lead tip, increasing the likelihood of complete extraction. Telescoping sheaths and laser sheaths provide counterpressure and countertraction, both disrupting fibrous tissue adhesion to vascular structures thus facilitating lead removal.
Life-threatening complications of lead extraction include myocardial avulsion, vascular tear, pneumothorax, pulmonary embolism, arteriovenous fistula, and death. Minor complications are pericardial effusion, hematoma, venous thrombosis, and arrhythmia. Recent reported complication rates are between 0.4% and 1.0% with lead extraction success rates of >95%. The most important factors predicting extraction failure are increased implant duration, physician inexperience, younger patient age, and ventricular versus atrial leads. The risk of failed extraction, based on this database, doubles for every 3 years of implant duration. Operators who have performed fewer than 20 extractions had lower success rates. As described earlier, the pediatric population is more likely to develop calcified fibrous matrices lending to lower success rates in younger patients. Because of the increased risks, such complicated procedures should be limited to experienced operators with all available resources including surgical operating room backup for emergency surgical intervention in the event of any of the above-mentioned life-threatening complications.
Pacemaker Follow-up Challenges
The objective of pacemaker follow-up is to optimize device function, prolong battery longevity, and identify possible lead/generator issues. At implant, threshold margins (both pacing and sensing) are programmed with 2 to 3× safety margin. This is because myocardial edema and scaring may transiently affect threshold values in a fresh lead implant. Lead maturation occurs over the course of 6 weeks to 3 months at which time thresholds remain stable (chronic thresholds) and reprogramming to optimize battery longevity is indicated.
Pacemaker status evaluation can be performed in a variety of settings. Conventionally, device checks are performed in the office by an electrophysiologist or a cardiologist with pacemaker programming
expertise. With the advent of remote monitoring, transtelephonic checks can provide information regarding sensing/pacing thresholds, stored electrograms in the device for arrhythmia troubleshooting, and battery longevity. However, formal device interrogations by a technician or an electrophysiologist are required on a yearly basis, and more often for those patients with CHD or ongoing pacemaker issues.
expertise. With the advent of remote monitoring, transtelephonic checks can provide information regarding sensing/pacing thresholds, stored electrograms in the device for arrhythmia troubleshooting, and battery longevity. However, formal device interrogations by a technician or an electrophysiologist are required on a yearly basis, and more often for those patients with CHD or ongoing pacemaker issues.
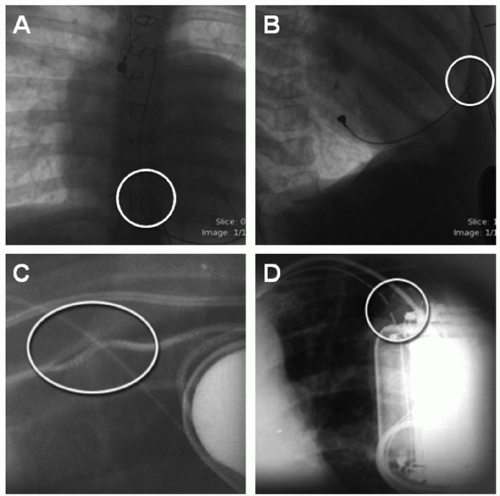 Fig. 107.9. X-rays in AP and lateral views showing epicardial (A and B) and transvenous pacing lead fracture (circles) (C and D). |
Generator Replacement
The commonest indication for generator replacement is battery depletion. Several factors influence battery longevity in pacemakers. Current drain for pacing is dependent on pacing rate, percent pacing, programmed voltage, programmed pulse width, and lead impedance. Ohm’s law (V = IR, V = voltage, I = current, R = resistance) dictates that the current is inversely proportional to impedance at any given voltage. Therefore, leads with higher impedances (such as transvenous bipolar leads) tend have lower current drain ( for a similar voltage) and longer battery longevity. Moreover, contemporary pacemakers have several features obligating current including sensing measurements, rate response sensors, and algorithm implementation.
Most devices are equipped with an algorithm that will estimate battery life. Devices that reach elective replacement indicator (ERI) will typically revert to an industrydictated mode that is recognizable to the clinician. For example, Medtronic devices will pace VVI at 65 bpm if ERI is reached. Depending on variables mentioned, generators will have only months of battery life at ERI and generator replacement planning during that time frame is required. Patients with CHD who are reliant on AV synchrony will become quite symptomatic in an asynchronous pacing mode (e.g., VVI). As such, many clinicians will opt to replace the generator just prior to ERI in such selected patients. Of course, a thorough investigation of lead competence should be performed prior to generator change in all patients.
Pacemaker Recalls/Device Malfunction
Pacemaker malfunction is rare, occurring in approximately 0.4% of all implanted pacemakers translating into 1.3 malfunctions per 1,000 person-years. Unfortunately, device and lead recalls carry an enormous financial and psychological burden. Regular monitoring of lead and device performance by various device companies and subsequent FDA reporting has paved the way for physicians to closely monitor some of the “recalled” leads and devices. As technology continues to improve, device malfunctions will hopefully be limited to nonclinically significant events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree