The percutaneous method of insertion of an intra-aortic balloon pump (IABP) through the femoral artery was introduced in 19791 and is performed usually in a cardiac catheterization laboratory, where optimal placement can be guided by fluoroscopy.2,3 Indications and contraindications for the procedure are outlined in Tables 15.1 and 15.2, accordingly.
TABLE 15.1Indications for intra-aortic balloon pump placement.
|
TABLE 15.2Contraindications to intra-aortic balloon pump placement.
|
Femoral Artery Approach
The right or left common femoral artery often serve as access sites of choice; on rare occasions, the left brachial access can be considered (Figure 15.1A). The balloon size is based on patient’s height: Patients taller than 183 cm receive 50-mL balloons, patients less than 162 cm receive 30-mL balloons, and all other patients receive 40-mL balloons. The balloon diameter, when fully expanded, should not exceed 80%–90% of the diameter of the descending aorta. As the tip of the needle is in the lumen of the common femoral artery, the 0.030-inch or 0.032-inch, J-tip guidewire is inserted and advanced through the needle into the descending aorta. Once the 7.5-Fr sheath is appropriately positioned, the side port of the sheath is connected to the manifold to record arterial pressure. A 60-mL syringe is connected to the balloon port, and the plunger of the syringe is slowly and completely withdrawn to create a vacuum within the balloon in order to minimize its bulk at insertion. The IABP central lumen is flushed with heparin, and it is advanced over the guidewire through the arterial sheath under fluoroscopic guidance into the aorta so that the radiopaque marker tip lies about 2 cm below the origin of the left subclavian artery or at the level of the carina, with the distal end above the renal arteries (usually corresponds to L1–L2 vertebrae). There should be no resistance to passing the balloon. Resistance usually indicates aorto-iliac disease, and in this case the balloon should be withdrawn and the aorto-iliac segment reassessed by angiography. Inflation of the balloon in this position should not cause occlusion of either the renal or subclavian arteries. The guidewire is withdrawn; the central lumen is aspirated and flushed with heparinized saline, and is attached to a pressure transducer.
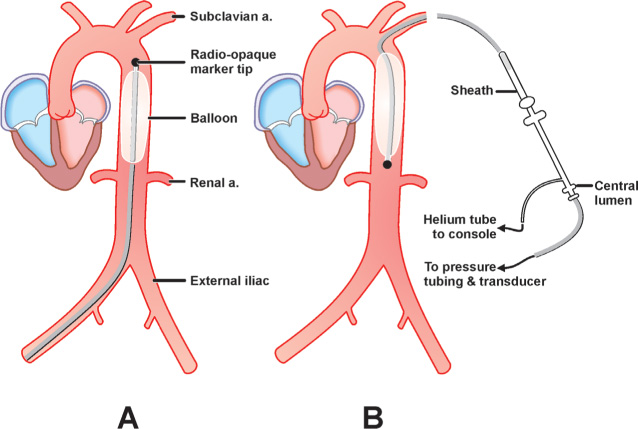
FIGURE 15.1Optimal positioning of the IABP is shown in (Panel A) the femoral artery approach and (Panel B) the left brachial artery approach.
The operator connects the balloon inflation port of the IABP catheter to the IABP console and fills the balloon with helium gas. The balloon should unwrap fully and there should be no kinks or filling defects. Complete filling of the balloon and its position should be verified by fluoroscopy. At this point, a cine image is obtained, and the angiographic frame stored.
When all these steps are completed, counterpulsation is initiated. A heparin bolus at 40 units/kg is given intravenously and a drip started at 12 units/kg/hour to keep PTT at 1.5-times control to reduce the incidence of thromboembolism. Distal pulses are checked, the proximal end is sutured securely to the skin and sterile dressing is applied. While the balloon is in position, the patient remains on strict bed rest with no hip flexion beyond 20 degrees. To obtain maximum hemodynamic effect from counterpulsation, it is crucial to optimally adjust the timing of balloon inflation and deflation. When adjusting timing of the balloon inflation and deflation, the operator places the balloon on a 1:2 counterpulsation sequence and observes the arterial waveforms of augmented and unaugmented beats from the catheter’s central lumen. Balloon inflation should immediately follow the closure of the aortic valve, coinciding with the dicrotic notch on the central aortic pressure tracing. Balloon deflation should be set to occur immediately prior to the aortic valve opening, which usually coincides with the “R” wave on the ECG tracing. Pacing spikes should be used to trigger the balloon in patients who are 100% paced. If the balloon functions well and timing is set correctly, the augmentation wave should be greater than the systolic pressure, and postdeflation aortic end-diastolic pressure should be 10–15 mm Hg lower than the same parameter of a nonaugmented beat (Figure 15.2C). After IABP insertion, peripheral pulses on both lower extremities must be checked regularly and frequently, and daily chest x-rays and general laboratory values (CBC, serum electrolytes, PTT) should be obtained.
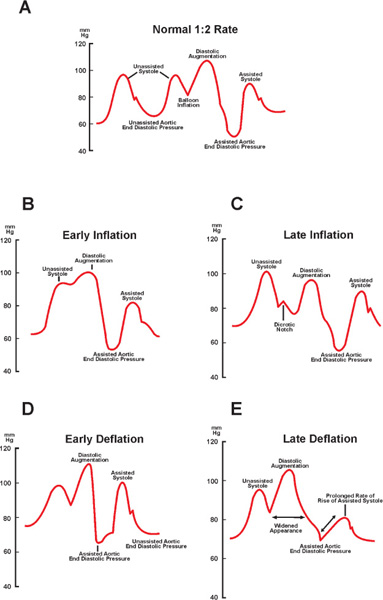
FIGURE 15.2Timing of inflation/deflation of the IABP (see text for details). Panel A: Normal aortic blood pressure tracing with optimal inflation of the IABP. Panel B: Abnormal aortic blood pressure tracing with early inflation of the IABP.Panel C: Abnormal aortic blood pressure tracing with late inflation of the IABP. Panel D: Abnormal aortic blood pressure tracing with early deflation of the IABP. Panel E: Abnormal aortic blood pressure tracing with late deflation of the IABP.
Left Brachial Artery Approach
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


