Chapter 99 Interventional Device Therapy in Atrial Fibrillation
Thromboembolism is the most feared complication in patients with permanent atrial fibrillation (AF).1 The risk of embolism may vary from less than 1% to 20%, depending on the underlying disease. Obviously, patients with previous strokes have the highest risk of embolism. Trans-esophageal echocardiographic (TEE) studies show that most thrombi are located in the left atrial appendage (LAA) and that only a few thrombi originate in the left atrium itself.2,3 Although prospective and randomized studies show that oral anticoagulation (OAC) reduces the risk of thromboembolism significantly, patients often may not receive OAC because of bleeding complications, increased bleeding risk, allergies to warfarin derivates, or fear of complications.4,5 Occluding the LAA is another way to reduce the risk of thromboembolism. Hence, the LAA is often occluded or removed in patients undergoing cardiac surgery with concomitant AF. Surgical obliteration of the LAA in patients with a high risk for thromboembolism is a well-established procedure to eliminate this predilection site for the development of thrombi.6–8
In 2001, an alternative procedure was introduced for patients with contraindications for OAC, that is, the interventional occlusion of the LAA.9–11 This chapter provides information on the indication, technique, safety, results, imaging, and potential future applications of percutaneous LAA occlusion.
Indication
Oral anticoagulation is contraindicated for at least an estimated 30% of patients with AF. Furthermore, it has been shown that contraindications for OAC are particularly common in older patients.5 Interventional occlusion of the LAA is suggested for patients with a high risk of embolism and a contraindication for OAC. Often the so-called CHADS2 score is used for defining the risk of embolism.
Devices and Implantation Techniques
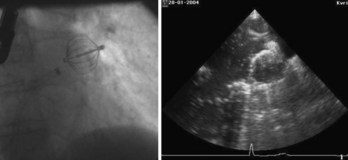
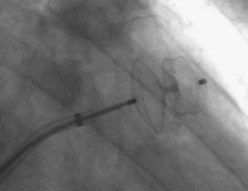
The first device for the interventional occlusion of the LAA was introduced by Lesh and co-workers (animal studies).9 The device was called PLAATO (percutaneous left atrial appendage transcatheter occlusion). It consists of a self-expanding, balloon-shaped nitinol cage with an expanded polytetrafluoroethylene (ePTFE) membrane with three rows of anchors stabilizing the device in the appendage (Figure 99-1). The membrane covers the atrial surface of the device, whereas the opposite surface is not covered, which allows secondary thrombosis of the lumen by the device. The morphology of the LAA is evaluated by angiography and simultaneous TEE. The maximal diameter of the LAA orifice is determined by both methods and a mean diameter obtained. A 20% to 30% oversized occlusion device is selected. The device is introduced via a 14-F femoral vein sheath and advanced to the LAA after trans-septal puncture of the atrial septum. Then, the device is expanded and the position is controlled using both angiography and TEE (Figure 99-2). The mean size of the devices used is 29 mm. After verifying the optimal position and adequate sizing of the device, it is released and the final position assessed by repeat angiography.
A similar device, called Watchman device, was introduced by Atritech (Plymouth, MN) (Figure 99-3).12 It is similar to the PLAATO device and consists of a self-expanding nickel-titanium frame structure with external fixation barbs and a permeable polyester fabric cover (sizes ranging from 21 to 33 mm). The procedure of implanting the device is similar to that of the implantation of the PLAATO device.
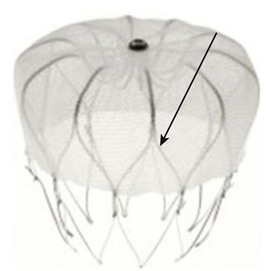
FIGURE 99-3 Watchman device (Atritech) with a self-expanding nitinol frame structure with external fixation barbs.
Meier and coworkers published a study on the successful occlusion of the LAA using an Amplatzer device, which was not designed uniquely for this purpose.13 In December 2008, AGA Medical (Plymouth, MN) received the European Conformite Europeene mark of approval for an LAA occlusion device that is different from the previously described ones. It is called a cardiac plug and is designed to achieve optimal occlusion of the LAA; it consists of a distal lobe and disc that is designed to cover the orifice of the LAA (Figure 99-4). The diameter of the lobe ranges from 16 to 30 mm, and the corresponding disc diameter ranges from 20 to 36 mm.
Safety and Results
In various studies, data about the safety and success of LAA occlusion were collected for both the PLAATO device (Figure 99-5) and the Watchman device.12,14
The researchers evaluating the PLAATO device showed that the procedure is feasible and may be performed within 2 hours.13 Detailed results concerning 111 patients with attempted LAA occlusion were published in 2005.13 The procedure was successful in 97% of the patients. Migration of the device or formation of mobile thrombi on the surface of the device were not observed during follow-up 10 months later. One of the patients in the study group died because of procedural complications. Pericardiocentesis was performed in three patients for cardiac tamponade. In addition, one patient developed left-sided hemothorax. Hence, relevant procedural complications were not uncommon. However, they occurred less frequently with increasing experience of the operator.
Two patients had a stroke, and two patients experienced transient ischemic attacks during the observation period. No thrombi formed on the device in any of the patients. Another study followed up the patients closely via echocardiography and showed that the implant was covered with a neointima after 6 months and that the device did not alter pulmonary vein flow.11 Furthermore, no significant atrial septal defects were detected after the puncture of the intra-atrial septum with an F-14 sheath.15 Because of the high rate of complications, a randomized study comparing anticoagulation and device therapy was requested. Such a study has not been performed yet, and the company has halted distribution of the device. Long-term follow-up data have not systematically been published yet.16–18
Similar experiences were reported for the Watchman device from Atritech. Sick et al provided information on 75 patients who had the device implanted.12 A failure rate of 10% was reported for implantation of the device. Two patients experienced device embolization. The devices were successfully retrieved percutaneously in both cases. Two cardiac tamponades and one air embolism were reported as well. One device had a wire fracture and needed to be explanted surgically. In four patients, flat thrombi had formed on the atrial surface of the device by the time of the 6-month echocardiographic follow-up. Two neurologic complications occurred during the follow-up period. To assess how effectively strokes and bleeding events are reduced in patients with AF by occluding the LAA with the Watchman device, the prospective Embolic Protection in Patients with Atrial Fibrillation (PROTECT-AF) study was performed in patients with nonvalvular AF and a CHADS2 score greater than 1.19 It is a key study for evaluating the concept of LAA occlusion for the prevention of thromboembolism in patients with AF, and hence the study will be discussed in detail in this chapter.
Other studies on LAA occlusion included far fewer patients. Meier et al provided information on the use of the Amplatzer device for occluding the LAA in 16 patients.13 One patient had an embolization of the device. All other patients had no serious complications.
Other devices are currently being assessed in animal models. Fumoto et al reported on the successful implantation of a third-generation atrial exclusion device in 14 dogs.20 Jayakar et al investigated a tissue welding technology to obliterate the LAA.21
Importantly, long-term data or data from randomized prospective trials are not available yet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree