CHAPTER 56 Interventional Cardiology
HISTORICAL PERSPECTIVE
After the refinement of diagnostic coronary angiographic catheters by Sones, Judkins, and Abrahms in the 1950s, the radiologist Charles Dotter performed the first endovascular dilation of a stenotic artery for therapeutic purposes in 1964.1 Peripheral arterial lesions were treated with progressive coaxial catheter dilation to improve blood flow. Complications including hematoma formation and distal embolization were common, and this technique, referred to as “dottering,” did not gain favor in the United States. However, European investigators continued to study and modify the technique. One such physician, Andreas Gruentzig, modified the Dotter multiple catheter system and developed a double-lumen catheter with a distensible balloon on the end that provided circumferential rather than coaxial pressure on the atherosclerotic plaque. However, inflation of the angioplasty balloon within the atherosclerotic plaque led to fissuring and cracking of the intima with stretching of the media and adventitia (Fig. 56-1).
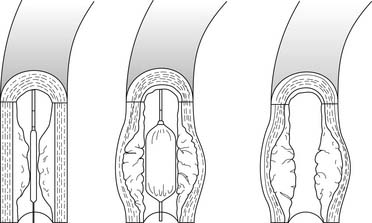
Figure 56–1 Mechanism of lumen enlargement with angioplasty.
(From Willerson JT, editor. Treatment of heart diseases. New York: Hower medical, 1992.)
Gruentzig performed the first peripheral balloon angioplasty in 1974 and then the first human coronary angioplasty in the operating room during elective coronary artery bypass surgery. When Gruentzig performed the first percutaneous transluminal coronary angioplasty (PTCA) in September 1977, the discipline of interventional cardiology was born.2
Initially, balloon angioplasty was offered as an alternative to bypass surgery in symptomatic patients with focal, proximal stenoses. Dilation of these arteries was usually associated with significant improvement in angina and in objective measures of ischemia.3 However, the application of PTCA was limited to a small fraction of coronary lesions, as first-generation fixed-wire balloons lacked the ability to be steered and the profile needed to traverse distal and tortuous vessels. Easier to use over-the-wire and rapid-exchange systems began to replace the initial fixed balloon-on-wire system developed by Gruentzig. The development of conformable, low-profile balloons facilitated dilation of distal lesions in tortuous and calcified vessels.
The widespread use of endovascular prostheses, or stents, unequivocally improved the acute safety and long-term efficacy of catheter-based treatment. As a result, over 1 million PCIs are now performed each year in the United States to alleviate symptoms and reduce adverse cardiac events.4 Stents were originally approved to seal dissection flaps and reverse acute vessel closure after PTCA by inhibiting elastic recoil in the arterial wall. The Gianturco-Roubin coil stent was the first such device approved in the United States, and it was approved on the basis of its ability to stabilize dissections and thus reduce the incidence of abrupt vessel closure and early complications.5 In 1994, the balloon-expandable Palmaz-Schatz slotted tube stent was approved for elective use after two randomized trials showed significant reductions in angiographic restenosis.6,7
PERCUTANEOUS CORONARY INTERVENTION PROCEDURES
Coronary intervention, like diagnostic angiography, begins with placement of a vascular access sheath in the femoral, brachial, or radial artery. Although choice of the access site is at the discretion of the operator, over 95% of interventional procedures are performed via the femoral approach.8 Radial or brachial arterial access may be preferable in certain circumstances such as iliofemoral occlusion, the presence of abdominal aortic aneurysm, recent femoral–popliteal bypass grafting, unfavorable body habitus, coagulopathy, or patient preference. Procedural success rates are comparable across all access sites, but radiation exposure and procedure times are slightly longer with brachial or radial procedures. Bleeding and vascular complications may be lower with brachial and radial access.9
Arterial access is obtained via percutaneous puncture of the anterior wall of the artery with a hollow needle.10 A guide wire is advanced through the lumen of the needle to the aorta, and the needle is removed. A vascular sheath is then inserted over this wire. Large-lumen guiding catheters are then advanced through the arterial sheath and over the guide wire to the ascending aorta. The guide wire is removed, and selective cannulation of the coronary arteries or bypass conduits is undertaken. If a stenosis is repaired, a 0.014-inch wire is used to traverse the stenosis, and the equipment used to treat the stenosis is advanced coaxially over this wire into the vessel (Fig. 56-2).
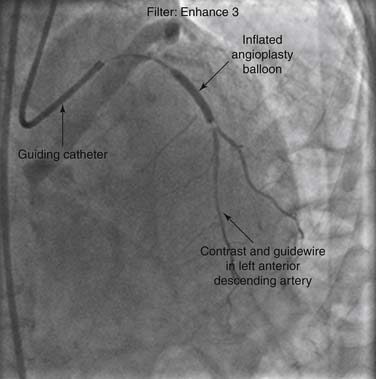
Figure 56–2 Angiogram during percutaneous coronary intervention of the left anterior descending artery.
PCI for Unstable Angina and Non-ST-Segment Myocardial Infarction
Current guidelines support the use of an early invasive management strategy for patients with unstable angina (UA) or non-ST-segment myocardial infarction (NSTEMI) who are at moderate or high risk for reinfarction or death, such as those with positive cardiac biomarkers, ST-segment changes on 12-lead electrocardiogram, or clinical parameters such as congestive heart failure, refractory angina, or arrhythmia.11 A recent meta-analysis supported the use of an invasive strategy in high- and low-risk men, and in women only if serum biomarkers were elevated.12 This strategy involves early administration of antithrombotic and antiplatelet medications as well as early angiography for further risk stratification. When the coronary anatomy is suitable, revascularization with coronary artery bypass grafting (CABG) or PCI is performed unless there is a strong contraindication. Typically, among moderate- and high-risk patients undergoing angiography for UA or NSTEMI, approximately 30% are managed without revascularization, 55% to 60% with PCI, and 10% to 15% with CABG.13 Those at low clinical risk can be treated with a selective invasive strategy, and revascularization can be used for those with high-risk noninvasive test results or recurrent symptoms. One large randomized trial found that monotherapy with the direct thrombin inhibitor bivalirudin was not inferior to the combination of heparin and a platelet glycoprotein (GP) IIb/IIIa receptor blocker for patients with acute coronary syndromes undergoing PCI.13
PCI for ST-Elevation Myocardial Infarction
The benefits of mechanical or pharmacologic reperfusion therapy in the setting of acute ST-segment elevation myocardial infarction (STEMI) are well established. PCI has been shown to effectively reestablish normal epicardial coronary perfusion in greater than 90% of patients. Long-term patency rates exceed 85%, and both the risk of reinfarction and death are significantly reduced with PCI compared with fibrinolytic therapy.1,14–16 Even if transport to a facility equipped for immediate PCI is required, catheter-based revascularization has been shown to have superior outcomes when compared with fibrinolytic therapy, assuming the artery can be opened expeditiously at an experienced center.17 Patients who develop cardiogenic shock in the setting of acute myocardial infarction (MI) also seem to benefit from immediate revascularization with PCI or CABG. Improved survival at 6 months has been shown in a randomized trial comparing immediate revascularization, using either PCI or CABG, with initial medical stabilization using fibrinolytic therapy or delayed revascularization (or both). However, in the subset of patients older than 75 years who had acutely infarcted, reduced survival was noted when immediate revascularization was used, compared with medical stabilization followed by delayed revascularization.18 Fibrinolytic therapy fails to restore normal blood flow in greater than 50% of patients, and PCI may also be indicated as salvage therapy in such cases. In those with persistent symptoms or persistent electrocardiographic evidence of ongoing infarction, “rescue” PCI has been shown to result in improved arterial patency with reductions in infarct size, early heart failure, and mortality.19,20
In comparison with PTCA alone, PCI with stent placement significantly reduces the incidence of death and ischemic target vessel revascularization, and the benefit is maintained for up to 5 years in patients with acute MI.21–23 Current guidelines support a 90-minute door-to-balloon time.24 Delays to reperfusion with PCI further decrease the mortality benefit of PCI over fibrinolysis, especially in higher-risk patients who present earlier in the MI period.25 The term facilitated PCI refers to the use of fibrinolytics with or without GPIIb/IIIa inhibitors prior to PCI. Facilitated PCI has been associated with improved angiographic results prior to PCI, but not after PCI, and also with a significantly increased risk of bleeding, without a proven mortality benefit.26 Therefore, there is not yet a proven beneficial role of facilitated PCI in STEMI.27
PCI for Chronic Stable Angina
Although medical therapy is preferred in patients with minimal symptoms and only a small area of ischemic myocardium, patients with large amounts of ischemic but viable myocardium in jeopardy have better anginal control if coronary revascularization is performed. Patients who are asymptomatic or whose symptoms can be controlled with medications, but who have high-risk noninvasive test findings have been shown to have lower rates of death or MI when treated with revascularization than when given medical therapy.28,29
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial found that in the initial management of stable coronary artery disease, PCI and medical management did not significantly differ in their reduction of death, MI, or adverse cardiovascular events.30 By 5 years, there was no significant difference in angina symptoms, although PCI was associated with earlier palliation of angina. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines for percutaneous coronary intervention, however, suggest that asymptomatic or minimally symptomatic patients with coronary lesions that are 50% stenosed or greater in one or two arteries undergo PCI if there is a high likelihood of success and a low risk of morbidity and mortality, and if the arteries supply a moderate or large area of viable myocardium.28,31 Thus, the decision to undergo elective PCI is based on the degree of myocardium at risk and failure of initial medical therapy.
Multivessel PCI
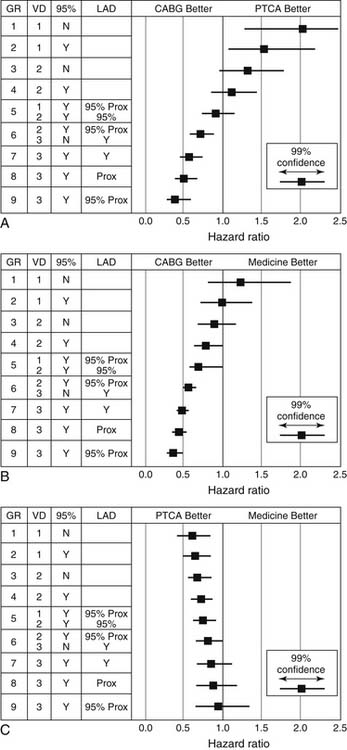
The comparative usefulness of multivessel PCI or CABG has been the subject of significant debate. Whether to perform PCI or CABG is often based on the severity and extent of disease, as well as on patient risk factors. Perceived benefits of CABG compared with PCI are described in Box 56-1. Various lesion subsets have been evaluated in registries that suggest a benefit for CABG compared with PTCA in patients with three-vessel disease or severe stenosis of the proximal left anterior descending (LAD) artery (Fig. 56-3).32 Randomized comparisons have been performed of CABG and PTCA (Table 56-1). The largest was the Bypass Angioplasty Revascularization Investigation (BARI) trial, which demonstrated that 5-year survival and survival free of Q-wave myocardial infarction did not differ significantly between the PTCA and CABG groups (86.3% versus 89.3%, and 78.4% versus 80.4%, respectively; P = .19). On the other hand, patients randomized to CABG were much less likely to require repeat revascularization. At 5-year follow-up, 54% of those assigned to PTCA had undergone additional revascularization procedures, compared with only 8% of the patients assigned to CABG. Nonetheless, 69% of patients initially assigned to PTCA avoided CABG over a 5-year period.33 All of the other trials comparing PTCA and CABG have shown similar results with a fourfold to 10-fold-higher likelihood of repeat revascularization after initial PTCA, but no significant differences in mortality or MI.
Box 56–1 Benefits of Coronary Artery Bypass Graft Surgery and Benefits of Percutaneous Coronary Intervention
Table 56–1 Randomized Clinical Trials Comparing Coronary Artery Bypass Grafting (CABG) and Percutaneous Coronary Intervention (PCI)∗
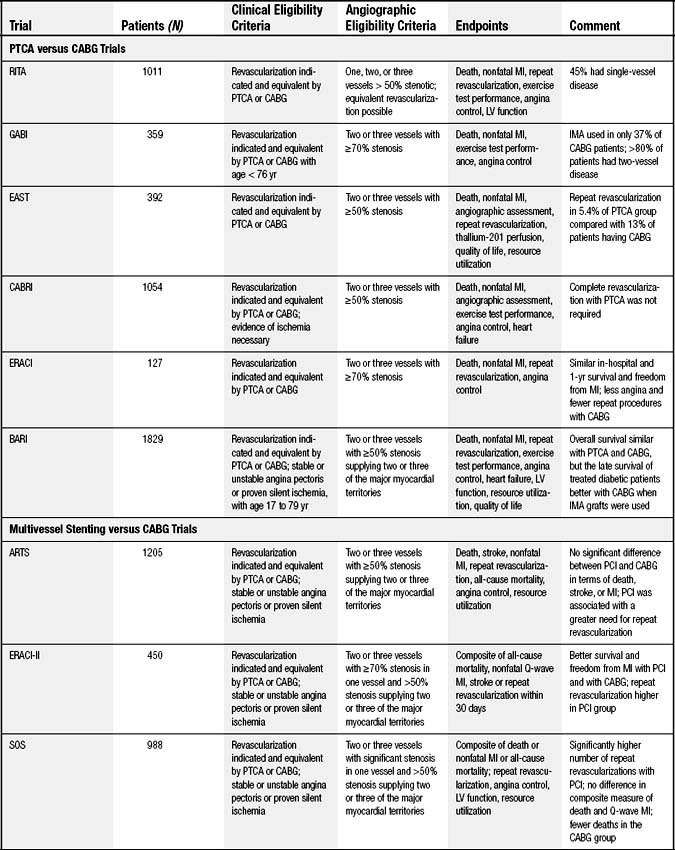
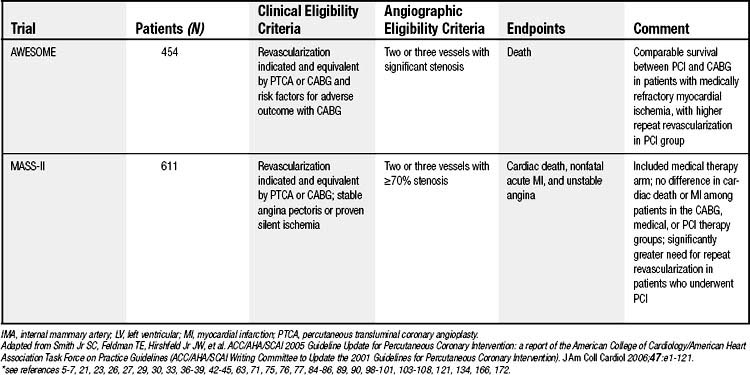
A meta-analysis of the randomized PTCA and CABG trials including 3371 patients (BARI was excluded as it was unpublished at the time)34 reported that there were no significant differences in mortality or MI. Rates of repeat revascularization were significantly higher with PTCA, particularly during the first year of follow-up. Rates of angina were also higher at years 1 and 3 with PTCA, but the relative advantage of CABG diminished over time.35,36
Although the randomized trials have not demonstrated differences in mortality between PTCA and CABG, CABG may provide an important advantage for diabetic patients.37 This finding is based primarily on the diabetic subgroup from the BARI trial (n = 353). At 5-year follow-up, all-cause mortality was 19.4% in the 180 patients assigned to CABG, and 34.5% among the 173 patients assigned to PCI (P = .003). Somewhat different results have been observed in registry studies, which provide important additional insight to the randomized controlled trials as only a very small percentage of all eligible patients were enrolled in the trials.38 For example, in a registry of patients who were screened but not randomized in the BARI trial, long-term follow-up demonstrated no significant difference in mortality between diabetic patients undergoing CABG and those assigned to PCI.39,39a In contrast to the randomized trial, patients in the BARI registry had their initial revascularization strategy chosen by their physicians.
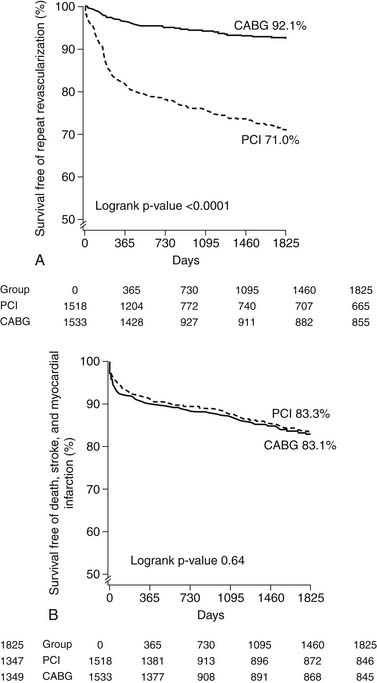
Several studies have examined outcomes of multivessel BMS placement compared with CABG (see Table 56-1). Meta-analysis of the four major randomized trials comparing BMS and CABG reveals a 5-year safety profile for PCI that is similar to that for CABG. Lower repeat revascularization rates were found among the CABG patients (29.0% versus 7.9%, respectively; hazard ratio, 0.23; 95% confidence interval, 0.18-0.29; P < .001) (Fig. 56-4, Panel A) with no difference in rates of death, stroke, or MI (see Fig. 56-4, Panel B). No subgroup, including diabetic patients and those presenting with 3-vessel disease, was found to have a selective benefit with CABG.40
It was postulated that reductions in repeat revascularization rates associated with the DES would lower adverse events after multivessel PCI sufficiently to favor this strategy over CABG. Nevertheless, the optimal treatment for patients with multivessel disease remains uncertain. Registry data suggest equivalent outcomes with the DES and CABG among nondiabetic patients but a continued advantage for CABG among diabetic patients.41 The Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial reported low repeat revascularization rates at 1 year among patients receiving the paclitaxel-eluting stent, compared with CABG.42 There was no statistically significant difference in risk of death (4.3% versus 3.5%, respectively; P = .37) or heart attack (4.8% versus 3.2%, respectively; P = .11). The risk of stroke was significantly greater for bypass surgery (0.6% for PCI versus 2.2% for bypass; P = .003). The combined endpoint of myocardial infarction, stroke, death, or revascularizaton was higher with PCI (17.8% versus 12.1%; P = .0015), mainly driven by differences in rates of repeat revascularization (13.7% versus 5.9%; P < .05).42 For patients with two- and three-vessel coronary artery disease, a large, observational registry from New York State found a lower incidence of death and MI for those undergoing CABG than for those receiving a DES.43 However, as with all observational studies, unmeasured confounders may have had an impact on the results. The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial is an ongoing study evaluating DESs or CABG for diabetic patients with multivessel disease.44
Left Main-Stem Disease
Stent placement in the left main coronary artery (LMCA) can be performed with high rates of procedural success. As is the case with multivessel PCI, PCI of the LMCA is associated with higher rates of repeat revascularization. The DES has rapidly and largely replaced bare metal stents for PCI of the unprotected left main. However, PCI is still chosen less frequently than CABG for unprotected left main revascularization. Registries describing outcomes after DES implantation or CABG for LMCA stenosis have demonstrated higher rates of repeat revascularization with PCI and lower mortality rates with CABG.43,45 It is unknown whether differences in patient risk and angiographic parameters affect these results. In most cases, CABG should be performed for those with LMCA disease who are candidates for surgery.46
Elective PCI and Noncardiac Surgery
The incidence of perioperative cardiac events ranges from 2% to 6%, depending on the type of surgery performed and the patient’s clinical risk factors. Most studies have focused on patients requiring vascular surgery. It is currently recommended that patients with three or more clinical risk factors and poor functional capacity (class IIa) may undergo further testing before vascular surgery. Those with one or two clinical risk factors and poor functional capacity undergoing intermediate-risk surgery or who have good functional capacity prior to vascular surgery (class IIb) may also undergo testing.47 The current ACC guidelines recommend PCI only for those who would receive a benefit of revascularization independent of surgery. Further cardiac testing is recommended prior to surgery only if it will change management.48 Moreover, surgery soon after PCI may be associated with adverse outcomes. Because uninterrupted antiplatelet therapy is necessary after stent implantation, bleeding complications may result in the perioperative period, or there may be catastrophic consequences caused by stent thrombosis resulting from discontinuation of antiplatelet medications or from a hypercoagulable state induced by surgery.
In a small cohort of 192 patients, the highest risk of cardiovascular events in the postoperative period occurred in those who discontinued clopidogrel prematurely after PCI (30.7% versus 0%, P = .026).49 Moreover, early surgery (at less than 6 weeks) after BMS placement has been associated with a higher incidence of adverse events such as death and MI50 and should be avoided when possible. Current guidelines suggest that after BMS placement, dual antiplatelet therapy (aspirin plus clopidogrel) should be administered for at least 1 month (or a minimum of 2 weeks if significant bleeding occurs), and for 3 months after sirolimus, and for 6 months after paclitaxel-coated stents, with a suggested duration of at least 1 year for all three (class Ib).28,31,51
COMPLICATIONS OF PERCUTANEOUS CORONARY INTERVENTION
Death
Mortality can occur during PCI from a variety of complications, including anaphylactoid reactions, acute MI, pericardial tamponade, stroke, or vascular trauma. Since stents have been used, mortality rates associated with elective coronary intervention have fallen to less than 0.3%.52,53 Patients at higher risk include older adults, those undergoing emergency PCI or PCI on saphenous vein grafts, and those with decompensated heart failure or reduced cardiac ejection fraction, end-stage renal disease, cardiogenic shock, or acute MI.14,54
Emergency Bypass Surgery
Although technical improvement made PTCA success rates reach approximately 60%,15 successful PTCA was limited by an inability to expand balloons in fibrocalcific lesions. In addition, the barotrauma associated with dilation of stenotic lesions may lead to medial dissection and many of the complications of angioplasty. Abrupt vessel closure occurs in approximately 5% of cases and is caused by a combination of factors including arterial dissection, vascular recoil, vasospasm, and thrombosis. Flow-limiting dissections and thrombosis were treated with prolonged balloon inflations and aggressive anticoagulation, but despite these treatments, emergency bypass surgery was required in almost 50% of cases of abrupt vessel closure. In the pre-stent era, emergency surgery was required in approximately 3% to 5% of procedures,15 with significantly greater morbidity and mortality than seen with elective bypass surgery.55–57 Cardiac tamponade may develop on occasion because of vessel perforation with the coronary guide wire or with balloon dilation.
The widespread use of stents has led to an evolution of the role of the cardiac surgeon in managing acute complications of PCI. During the initial development of PTCA, immediate surgical backup was necessary, because not infrequently, acute vessel closure necessitated urgent surgical revascularization. The incidence of emergency cardiac surgery for failed PTCA was approximately 5% to 7% in the 1990s.58,59 The need for emergency surgery has fallen dramatically in recent years, however, paralleling improvements in stent technology and pharmacologic therapy, and it now occurs in less than 1% of cases.18,60 Registry data support the safety of elective PCI in community hospitals without the presence of cardiac surgery backup for low-risk patients.61,62 Although the incidence of emergency surgery is low, the ACC/AHA guidelines for PCI do not support the performance of elective PCI without surgical backup.28 A large, prospective trial, MASS-COMM, is currently randomizing patients in Massachusetts to PCI in a hospital without on-site surgical backup versus PCI at an institute with on-site surgical backup.
The improvements in immediate PCI outcomes and the recognition of improved survival with PCI performed in the setting of acute MI have led to the development of programs performing primary PCI without surgical backup. The C-PORT study confirmed the safety of primary PCI without surgical backup.63
It is well known that operator experience and hospital volume play an important role in these cases, as PCI for acute MI is often more technically demanding than routine PCI. Various studies have correlated both limited operator experience (<75cases/yr) and low hospital volume (<200 cases/yr) with less favorable outcomes for primary PCI.64–66
PCI for acute MI at institutions without surgical backup is currently performed, and published guidelines consist of requirements for operator and hospital volume, ready availability of necessary interventional and hemodynamic monitoring equipment, rigorous quality assurance, and a formalized protocol for immediate (<1 hour) transfer for emergency CABG.28 Certain lesion subsets that would ordinarily be attempted by operators with onsite surgical backup are proscribed when surgical backup is not available. Because of the risk of catheter injury to the left main artery, culprit stenoses (i.e., occluded 60% or more) that are downstream from a lesion in the left main coronary artery should be avoided. When there is normal flow in the infarct artery, and either the patient has three-vessel disease or the culprit lesion is long or angulated, PCI should be avoided as well. High-grade left main coronary disease or hemodynamic instability should prompt rapid transfer to an institution equipped to perform bypass surgery, preferably after placement of an intra-aortic balloon counterpulsation device.28
Emergency bypass surgery is performed mainly for persistent abrupt vessel closure, extensive vessel dissection, unstable left main coronary artery stenosis or injury, uncontrolled vessel perforation, and cardiac tamponade. The Society of Thoracic Surgeons reported an operative mortality that exceeds 5% for patients requiring emergency bypass surgery within 6 hours of PTCA.60,67 Although the frequency of emergency surgery continues to fall, and surgical technique and postprocedure management continue to improve, patient outcomes continue to be significantly worse than for elective surgery. The poor outcomes in this population are not unexpected, because as PCI has improved, the patients who cannot be salvaged and require emergency surgery comprise a population with more unfavorable risk factors and comorbidities. Considerations such as conduit selection, myocardial protection, and the need for coronary artery repair make the surgical procedure technically demanding.
Myocardial Infarction
Although Q-wave MI occurs in less than 1% of patients, the incidence of postprocedure myonecrosis (as measured by elevation of cardiac markers such as creatinine phosphokinase [CPK]-MB and troponins) may be found in almost one third of patients and is more common after atherectomy procedures, and after PCI performed in thrombus-containing lesions, in the setting of acute coronary syndromes, or for treatment of saphenous vein graft disease.68–70 Periprocedural myocardial infarction may result from acute vessel closure, perforation, or, most commonly, embolization of thrombus or atheroma.
Vascular Complications and Hemorrhagic Complications
The use of antithrombotic, fibrinolytic, and antiplatelet agents, as well as aggressive anticoagulation and prolonged sheath placement is associated with an increase in vascular complications.71 Impaired coagulation and the need for larger-lumen catheters for coronary intervention (typically 6 to 8 French) lead to more frequent access-site complications compared with simple diagnostic angiography. Complications include hematoma (1% to 5%), retroperitoneal hemorrhage, and pseudoaneurysm (1%) formation.72 Patients with significant peripheral vascular disease are at higher risk for such complications. Infrequent (<1%) complications include arteriovenous fistula formation, infection, cholesterol embolization, and vascular occlusion. Femoral access-site complications requiring surgical repair or transfusion occur in 2% to 5% of cases. Traditionally, surgery was performed to close pseudoaneurysms greater than 2 cm in diameter because of significant risk of spontaneous rupture. Ultrasound-guided injection of thrombin into the pseudoaneurysm has obviated the need for open surgical repair. Surgical exploration is undertaken for repair of fistulae and arterial lacerations, and in cases of arterial occlusion and uncontrolled bleeding leading to hemodynamic compromise.
Contrast Nephropathy
Nephropathy induced by contrast media used for angiography is an important clinical concern. The occurrence of contrast agent–induced nephropathy is associated with poorer clinical outcomes.73,74 Although most of these cases are self-limited, a fraction of patients require short-term dialysis and some require permanent dialysis. The risk of occurrence is less than 1% overall. Patients with baseline renal insufficiency or diabetes, or those who receive large volumes of contrast material are at highest risk. The risk in this population is more than 35%.73,74 Additional risk factors include dehydration, advanced age, large contrast volume, multiple myeloma, exposure to nephrotoxic drugs, congestive heart failure, and liver disease.
The risk of contrast agent–induced nephropathy can be reduced by hydration, with either normal saline or sodium bicarbonate, before and after the procedure. Forced diuresis, calcium antagonists, dopamine, and atrial natriuretic peptide have not been shown to be beneficial.75,76 The use of an iso-osmolar contrast agent, iodixanol, was compared with a low-osmolar contrast agent, iohexol, in high-risk patients, and was shown to reduce the incidence of contrast-induced nephropathy.77 There is evidence that acetylcysteine, selective dopamine-1 receptor agonists, and theophylline may be beneficial in some cases, but the role for these agents requires further study.75,78,79
Anaphylactoid Reactions
The incidence of death from contrast media complications in the catheterization laboratory is estimated at 1 death per 55,000. Mild cases may be associated with a variety of symptoms such as itching, urticaria, flushing, cough, sneezing, wheezing, abdominal cramps, diarrhea, headache, back and chest pain, nausea, vomiting, fever, and chills. More severe reactions lead to dyspnea, a sense of impending doom, and hypotension, potentially resulting in cardiovascular collapse and death. Depending on the severity of the clinical findings, patients may require treatment with catecholamines, antihistamines, corticosteroids, or intravenous fluids. In patients with prior reactions, pretreatment with corticosteroids and diphenhydramine, and the use of nonionic contrast media have significantly reduced the potential of recurrent reaction.80
Restenosis
Although angiographic restenosis may be silent clinically, many patients present with recurrent angina or provocable ischemia during functional testing. It is now recognized that restenosis may not be clinically benign: about 10% of patients with restenosis present with acute MI.81 Risk factors for restenosis after PTCA have been well documented (Table 56-2). In large populations, late lumen loss follows a near-Gaussian distribution, with approximately 50% of the initial gain in lumen diameter lost by 6 to 8 months.82 The slope of the gain–loss relationship is approximately 0.5, and it is similar across all devices used for PCI. The reduction in restenosis rates observed after BMS placement is entirely explained by the ability of these stents to safely maximize acute gain, thereby allowing greater tolerance for late loss. The DES also decreased neointimal and smooth muscle cell growth, reducing late loss as well, in addition to the greater acute gain seen with the BMS.
Table 56–2 Risk Factors for Restenosis
| Patient Factors | Lesion Factors | Technical Factors |
|---|---|---|
| Diabetes | Small vessels | High residual post-treatment stenosis |
| End-stage renal disease | Left anterior descending location | |
| Unstable coronary syndrome | Long lesions | |
Nevertheless, restenosis remained a problem in interventional cardiology, with rates ranging from 12% to greater than 50% in certain subgroups with the BMS (see Table 56-2).17,83 The advent of the DES introduced local drug delivery from a polymer-coating on the stent consisting of anti-inflammatory and antiproliferative medications with a slow controlled release that effectively reduced the incidence of restenosis from 30% to less than 10%.84–86 Restenosis after DES placement is generally treated with repeat PCI, with or without another DES, or with CABG.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree