4
Intervention for chronic lower limb ischaemia
Introduction
This chapter will focus upon intervention for chronic lower limb ischaemia. Exercise therapy is considered in Chapter 3. The decision to treat patients with chronic lower limb ischaemia by endovascular or open surgery will depend upon the severity of symptoms, associated comorbidity, technical considerations and evidence-based outcomes. Open surgery is usually reserved for patients in whom conservative or endovascular treatments are not an option or where they have failed to produce significant relief of symptoms. The basic principles of open vascular surgery include the identification of an adequate inflow, a suitable conduit and adequate run-off. Patients must be made aware that any intervention runs the risk of failure or other complications, which may result in limb loss and/or death.
Patient selection
The symptoms of patients with chronic lower limb ischaemia can range from mild claudication to limb-threatening ischaemia. The decision to intervene in claudication will depend on walking distance, the patient’s current lifestyle and their desire to regain their loss in quality of life. This has to be reviewed against the potential risk of any intervention, the long-term durability of the procedure and the need for further intervention. For instance, younger patients who claudicate at 400 metres might be severely handicapped if they can no longer work effectively or take part in leisure activities. Conversely, elderly patients with a claudication distance of 50–100 metres might not be particularly handicapped if they have good access to local amenities and a supportive family. These two scenarios highlight the importance of considering quality of life in the treatment equation. Box 4.1 summarises the factors influencing the decision to intervene in claudication.
The CLEVER trial1 randomised 111 patients to best medical care (BMC) alone, BMC plus supervised exercise or BMC plus iliac stenting. At 6 months those patients who received BMC plus supervised exercise had a significantly better treadmill walking distance than those who had received BMC and stenting, and both did significantly better than the group receiving BMC alone. However, the patients who had stents inserted reported significantly better quality of life than those who received supervised exercise. This difference in end-points was not explained but is likely to be a placebo effect of perceived treatment over non-treatment if the patients fail to see supervised exercise as an intervention.
The MIMIC trial assigned patients with the relevant level of claudication to angioplasty plus best medical therapy plus supervised exercise or supervised exercise plus best medical therapy alone. Thirty-four patients with aorto-iliac disease and 93 with femoropopliteal disease were randomised. Both the absolute walking distance and the claudication distance improved by 30% more in the femoropopliteal groups and by 78% more in the aorto-iliac group in the angioplasty arm of the study.2 Though criticised as a small trial it is significantly larger than the often quoted other randomised controlled trials (RCTs) from Oxford and Edinburgh,3,4 which randomised patients to angioplasty or exercise and found that exercise was more effective. Also, the degree of improvement in the angioplasty arm would have made it difficult ethically to continue the MIMIC study.
Unlike patients with intermittent claudication, most patients with critical limb ischaemia (CLI) require some form of intervention. There are some situations when this would be inappropriate and these patients should not undergo unnecessary investigation or treatment. If the general condition of the patient is poor, and the chances of survival limited due to coexistent pathology, the involvement of a palliative care team is recommended (Fig. 4.1). However, if the patient has a reasonable quality of life and is likely to survive for more than a few months, it is probably better to attempt revascularisation whenever possible. No one wishes to see a patient spend the remaining few months of life struggling with an amputation as well as their primary disease. Some patients present with such advanced ischaemia that there is little viable tissue left on the weight-bearing area of the foot. It is sometimes possible, with revascularisation to the relevant angiosome and a customised forefoot amputation, to achieve a functional foot, but if this is not possible a primary amputation is the better option (see Chapter 5). The chair- or bed-bound patient can provide a challenge when deciding on whether to intervene. Fixed flexion deformities may also make amputation the best option. However, the rehabilitation team should be involved in this decision, as the patient’s ability to transfer from a chair to the bed or toilet may depend on preservation of that limb.
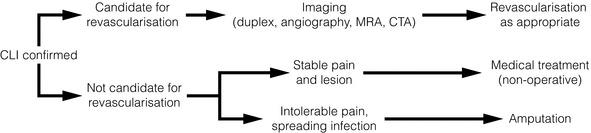
Figure 4.1 Algorithm for treatment of patients with critical limb ischaemia. CTA, computed tomography angiography; MRA, magnetic resonance angiography. Adapted from Norgren L, Hiatt WR, Dormandy JA et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45(Suppl S):S5–67. With permission from the Society for Vascular Surgery.
Preoperative independence and mobility have been shown to be good predictors of postoperative independence and mobility after infrainguinal bypass for CLI.5 Only one of 25 survivors who were not living independently before surgery achieved independent living 6 months postoperatively. Therefore, there seems little point in undertaking extensive revascularisation in the hope of achieving independence for a patient already requiring community care.
Cost-effectiveness
The cost-effectiveness of any intervention requires careful evaluation. A retrospective analysis of CLI in elderly patients found that the hospital cost of reconstruction was nearly twice the cost of primary amputation.6 However, if the community costs were added, amputation was more than twice as expensive, partly because 66% of reconstructed patients were able to return home compared to only 33% of amputees. A more recent prospective study also found that amputation was more expensive than revascularisation at 1 year.7 There was little difference between the cost of successful endovascular treatment compared with surgical reconstruction, because the length of inpatient stay was related more to the state of the foot than the treatment received.
A prospective study of 150 patients with CLI found that activities of daily living and pain scores improved significantly after successful reconstruction and primary amputation, but mobility was only improved by successful reconstruction.8 Thus, the improvement in the overall quality of life of those undergoing successful reconstruction seems largely due to better mobility. Patients whose reconstruction fails, leading to secondary amputation fare badly, both in terms of quality of life and cost, emphasising the need for a high success rate if undertaking this type of reconstruction. This is especially true for infrainguinal bypass procedures. In one study, the surgical ideal of an uncomplicated infrainguinal bypass operation, with rapid relief of pain, swift wound healing, a rapid return to premorbid function and no further intervention, was only achieved in 16 of 112 patients.9
TASC recommendations
There are differences between these recommendations but these are not particularly significant and they are summarised in Table 4.1. The recommendations have been updated to account for advances in endovascular techniques. Both classify peripheral arterial disease according to the level of the disease (e.g. aortic, iliac, femoropopliteal or crural) and by the severity of the disease (e.g. types A–D). As the disease becomes more severe and/or more diffuse, the recommendations move from endovascular to open intervention:
Table 4.1
Endovascular therapy or open surgery: summary of recommendations of the TASC II Working Group
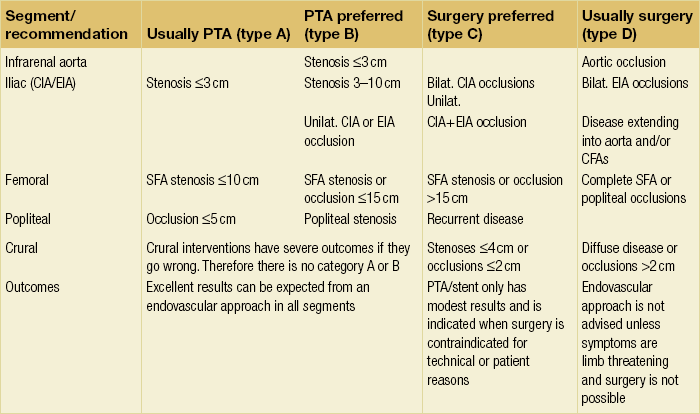
• type A lesions should usually be treated by endovascular means;
• type B lesions should preferentially be treated by endovascular means;
• type C lesions should preferentially be treated by open revascularisation;
Suprainguinal endovascular intervention
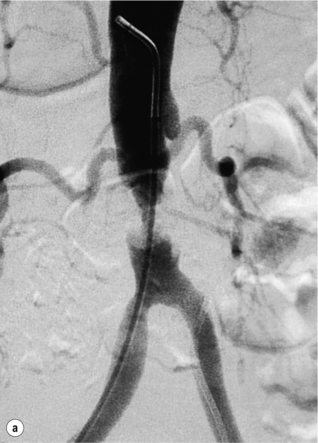
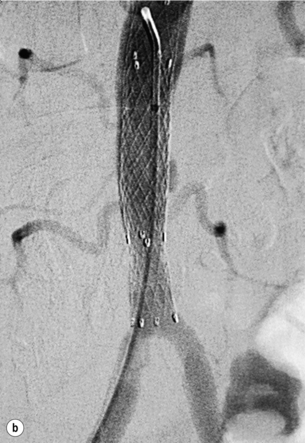
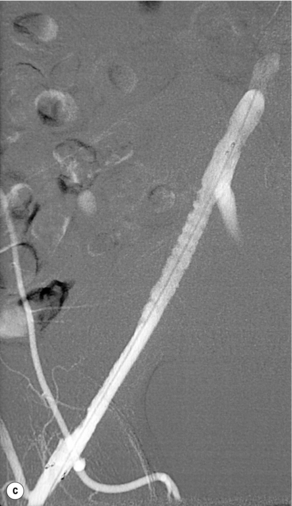
Haemodynamically significant stenoses of the infrarenal aorta are rare and usually seen in short, obese female smokers with a small-calibre aorta. Prior to the advent of endovascular therapies these patients would have undergone a localised aortic endarterectomy but primary stenting is now the first-line treatment (Fig. 4.2), with 5-year patency rates of 50%.12 Complications are rare and usually relate to embolisation, pseudoaneurysm formation and recurrent disease.13 Complete aortic occlusions are best treated by an aorto-bifemoral bypass (see later). In an unfit patient aorto-uni-iliac stenting, combined with a femorofemoral crossover, may be a better alternative to an axillo-bifemoral bypass.14 Kissing stents can be used but there is a risk of one stent compressing the other.
Iliac arteries
Though the European guidelines on the management of CLI and the diabetic foot18 state that there are no randomised studies of percutaneous transluminal angioplasty (PTA) to open surgery in the iliac arteries, this is incorrect.
There is one RCT that compares open surgery to angioplasty in symptomatic iliac disease.19 In this study, 263 men with iliac disease that was a major component to either rest pain or lifestyle-limiting claudication underwent either bypass surgery (n = 123) or angioplasty (n = 129). There were three deaths in the surgery group and none in the angioplasty group. There was no difference in patency and limb salvage rates, at a median follow-up of 4 years. The authors concluded that the lower morbidity and mortality rate in the angioplasty group supported the concept of angioplasty first strategy as outcomes between the two treatments were no different. The study was, however, limited by (i) the absence of women, who have smaller arteries and (ii) the relatively small numbers, which precluded subgroup analysis.
In a second, non-randomised comparative study the complication rate, primary patency and cost of stent deployment were compared with direct surgical reconstruction for the treatment of severe aorto-iliac occlusive disease.20 Sixty-five patients underwent stent deployment and 54 patients had surgical reconstruction. No significant difference was observed between the groups in terms of clinical presentation, demographics and late complications. The cumulative primary patency rate for bypass grafts was, however, significantly better than for iliac stents at 18 months (93% vs. 73%), 30 months (93% vs. 68%) and 42 months (93% vs. 68%). Multivariate analysis suggested that females, anyone with ipsilateral superficial femoral artery (SFA) occlusion, and patients who had procedure-related vascular complications and hypercholesterolaemia were more likely to thrombose their bypass graft or stent. Costs did not differ significantly.
When is an iliac lesion significant?
The arteriographic grading of stenoses is hampered by wide intra- and inter-observer variability.21 Intravascular ultrasound provides better measurements of cross-sectional area but it is expensive and not widely available. In theory, pressure gradient measurements at the time of angiography should be a relatively simple procedure. However, there is no consensus in the literature regarding what constitutes a significant pressure gradient22 and there is little or no information on the role of vasodilators (doses), systolic versus mean pressures and peripheral resistance in assessing stenotic vessels. Tetteroo et al. reported that they would have stented between 4% and 87% of their patients, emphasising the overall lack of consensus.23 They suggested that a mean gradient of 10 mmHg at rest or after vasodilation should be used as a marker of significance and this is generally accepted, though there is no evidence at all for this.
Who should have an angioplasty or a stent?
Early aorto-iliac angioplasty had a high rate of embolic complications, particularly when dealing with occlusive rather than stenotic disease.24 As a result stenting became the first-line treatment of iliac occlusive disease based on this observation. RCTs have been performed, but these have been compounded by flawed design and study methodology. Such a study demonstrated 4-year patency rates of 91.6% for stents and 74.3% for PTA alone, but the results have never been fully published.25
There is only one peer-reviewed, published RCT that compares primary stent placement with PTA followed by stent placement if needed in the iliac arteries. The Dutch Iliac Stent Trial (DIST)26 randomised patients to either a primary Palmaz stent or PTA alone. In the latter group stents were reserved for patients with suboptimal PTA results. The authors reported that 43% of patients randomised to PTA received iliac stents and that 2-year cumulative patency rates for the two groups were similar at 71% versus 70%. They concluded that because angioplasty followed by selective stent placement is less expensive than primary stent placement, the former should be the treatment of choice for lifestyle-limiting intermittent claudication caused by iliac artery occlusive disease. However, only 29 iliac artery occlusions were treated among 279 patients and, by study design, patients with occlusions more than 5 cm in length were ineligible for enrolment. PTA alone failed in 10 out of 12 subjects (83%). Although stents seem to offer special benefits in the treatment of longer segment lesions, little guidance about such patients is provided by this trial. Furthermore, most patients in the DIST study had mild clinical symptoms, and only 22% of patients in each group were classified as having Society for Vascular Surgery/International Society for Cardiovascular Surgery (SVS/ISCVS) grade 3–5 ischaemia. As a consequence, the milder pattern of atherosclerotic disease observed in the DIST study differed from that encountered in many interventional practices. In addition, the study was designed to have a 90% likelihood of detecting a 10% difference in post-treatment arterial patency after 12 months between the two groups (power = 90%). Unfortunately, the trial was terminated after less than 80% of the intended sample had been enrolled. Outcome information after as little as 1 year is available in the published report for about 60% of subjects who ultimately enrolled, a fraction that actually represents less than 45% of the intended patient sample. Thus, even though the authors did not observe ‘substantial differences’ in clinical outcomes between their experimental groups, the DIST study was more likely to miss than to detect the same potential 10% improvement in 12-month patency rates that was used to configure the trial, as the actual power was less than 50%. Because of under-recruitment, the authors attempted to amplify their sample by reporting the number of lesions treated, rather than the number of subjects actually involved. For example, a patient with simultaneous common and external iliac stenoses or occlusions was classified as two treated lesions, rather than as a single person. As a result, little information about crucial patient subgroups (e.g. occlusions vs. stenoses, common iliac vs. external iliac lesions) can be gleaned from this report. Despite these problems the authors continued to publish from the original dataset, reporting patency rates and patient survival at 5 years.27
There is a second RCT of stent versus angioplasty in complete iliac occlusions (the STAG trial). This is not yet published in the peer-reviewed literature but has been presented.28 The results suggest that there are significantly fewer major complications, particularly embolisation, following stent compared to PTA, but that stents confer no benefit in terms of patency (P. Gaines, personal communication). If so, we are brought full circle to the original premise that stenting infers benefits in terms of reduced complications in iliac occlusions, rather than improved patency. Though the authors are not in a position to comment until the publication of this study, much will depend on the original design and power of the study in terms of its primary and secondary end-points.
Iliac stenoses
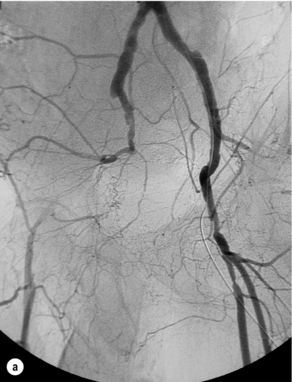
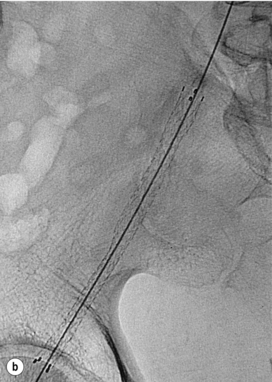
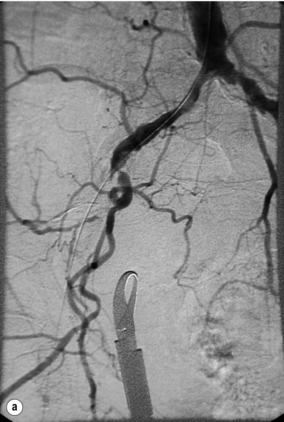
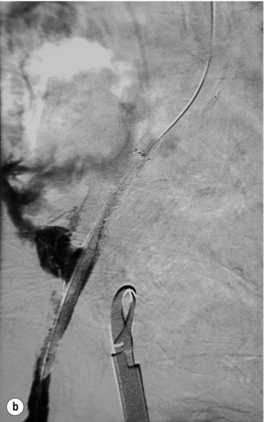
In iliac stenotic disease, angioplasty alone should be the procedure of choice. The end-point for aorto-iliac revascularisation procedures is determined by intra-arterial haemodynamic measurements, which are readily obtained in this arterial segment. Ideally, simultaneous waveforms obtained above and below the treated segment should overlap, with no mean or systolic gradient (Fig. 4.3).
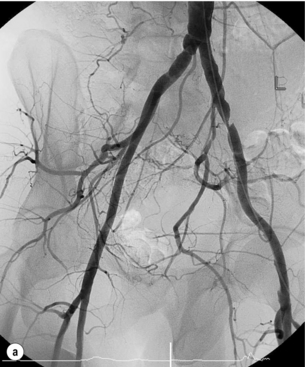
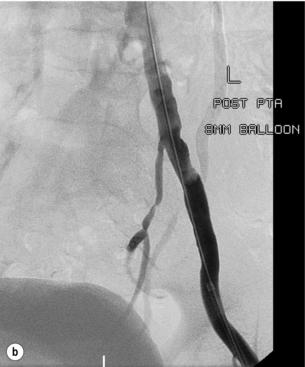
Figure 4.3 (a) There is a severe focal stenosis of the left external iliac artery. (b) After balloon dilatation there is minor residual stenosis. However, there is no pressure gradient across the lesion and stenting is not required.
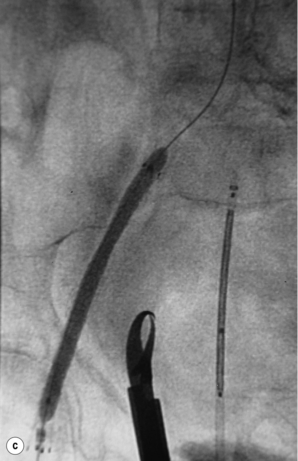
Iliac occlusions
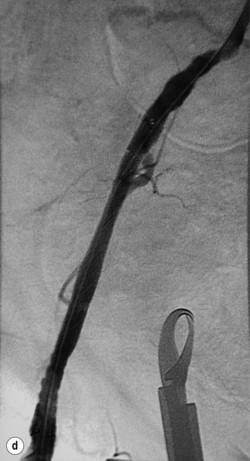
Chronic iliac occlusions can be crossed from the ipsilateral or contralateral side and stented to reduce the risk of embolic complications. This requires the insertion of a stent prior to PTA (Fig. 4.4). Self-expanding stents are indicated and in the authors’ experience this will always traverse the chronic occlusion over the wire. Heparin is mandatory immediately after arterial access. Once a stent of appropriate diameter and length has been deployed it can then be inflated with a suitable sized balloon. If there is a suitable iliac stump of the ostium, a uni-iliac stent will work well. If, however, the occluded iliac artery does not have a stump and the aortic lumen tapers smoothly to the occlusion, then ‘kissing’ iliac stents may have to be used, even when the contralateral iliac artery does not have a significant stenosis.31 This should be avoided if at all possible. Early results are good32 but, in small arteries, the long-term patency is not as good as uni-iliac stenting. The external iliac artery is slightly more prone to rupture than the common iliac, but responds well to stenting.
Complications
The third British Iliac Angioplasty Study (BIAS)33 followed 2233 patients for 12 months to 8 years. The complication rate in patients suffering intermittent claudication was 2.6% and in patients with critical leg ischaemia was 11.6%, reflecting the difference in age, comorbidity and disease extent. However, only 2.4% of patients overall required unplanned surgery, unplanned endovascuscular intervention or some other procedure that delayed discharge from hospital. The overall mortality for inpatient intervention was 2.8% and for day-case intervention no deaths were reported. Importantly, arterial perforation remains rare, with an overall mortality of 0.17%.
Potentially serious complications include the following:
Bleeding and pseudoaneurysm development
This can be treated by direct digital pressure but if there is a pseudoaneurysm, ultrasound-guided compression can be used,34 though thrombin injection, endovascular stent graft or open surgery may be necessary.35
Arterial rupture
Often, when rupture occurs during PTA, the patient experiences severe pain. But rupture has occurred without pain or other symptoms; therefore, it is very important to perform an arteriogram immediately after angioplasty. Gross extravasation of contrast is generally seen in cases of frank rupture (Fig. 4.5). Once an arterial rupture is recognised, rapid action is required to prevent death. It is always wise to ask for help from a colleague vascular interventionist. Tamponade of the leak with the angioplasty balloon followed by a covered stent graft to seal the rupture is the most appropriate treatment. Resultant hypotension and bradycardia should be treated with resuscitative therapy.36–38
Embolisation
Embolisation occurs in 2–7% of patients,33 and is more common in occlusive disease and in patients with CLI. The nature of the emboli (plaque, thrombus or cholesterol) determines the success of the different therapeutic options. Local thrombolytic infusion works poorly if the embolus is solid material (plaque). Suction thrombectomy is good for either thrombus or plaque. It requires a non-tapered catheter with a large end hole. Occasionally, large emboli have to be surgically removed.
Stent-related complications
Device-related problems such as (i) failure to deploy or misplacement and (ii) infection are rare and beyond the scope of this chapter.36 Patients with infected stents should, however, be started on intravenous antibiotic therapy and investigated with a view to removal of the stent with revascularisation using autologous vein, preferably via an extra-anatomical route.
Suprainguinal open surgical intervention
The surgical approach to patients with significant aorto-iliac disease is determined by the patient’s general fitness and whether or not they have had previous abdominal surgery (Fig. 4.6). In general terms all patients should undergo cardiorespiratory assessment, e.g. respiratory function tests, left ventricular ejection fraction (echo or multiple gated acquisition scan, MUGA), and preferably a cardiopulmonary exercise test. If they can exercise to a VO2 peak that implies an adequate reserve for surgery (> 15 mL/kg/min), further investigation may not be required. However, if they cannot exercise due to claudication pain then a dobutamine stress echocardiogram or Myoview scan might be more appropriate.39
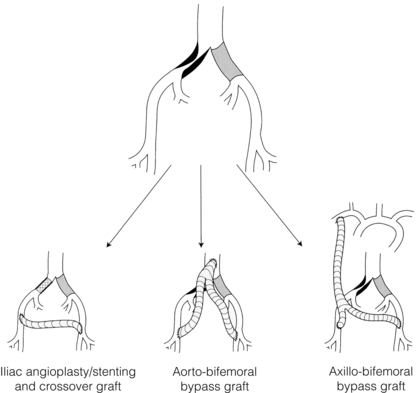
Figure 4.6 Possible treatment options in a patient with aorto-iliac disease. Axillo-bifemoral bypass should be reserved for patients with critical ischaemia.
Aorto-bifemoral bypass
The infrarenal abdominal aorta can be approached laparoscopically or by conventional open surgery. Laparoscopic aortic surgery has been introduced in many European countries and advocates claim lower morbidity and mortality rates and shorter hospital stays.41,42 There remains no convincing evidence to support these claims as there have been no randomised trials. Laparoscopic aortic surgery requires extensive training and the procedure times are longer than for open surgery. With regard to open surgery, there is little or no evidence to favour either a transperitoneal versus a retroperitoneal approach or a vertical versus transverse incision, although transverse incisions are favoured by anaesthetists as the top end of a vertical incision often ‘escapes’ an epidural. End-to-end anastomoses are used for flush occlusions below the renal arteries (Fig. 4.7). If the proximal infrarenal aorta is patent, then an end-to-side anastomosis can preserve flow to a patent inferior mesenteric and/or internal iliac artery, thus reducing the risk of pelvic ischaemia.
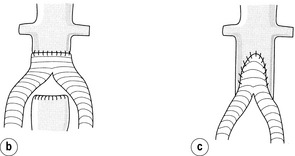
Figure 4.7 Aortogram showing occlusion of the aorta below the level of the renal arteries (a). In this case the proximal anastomosis of an aorto-bifemoral bypass should be end to end (b). If there is continuity with the inferior mesenteric artery or internal iliac arteries, then an end-to-side anastomosis (c) may be preferable.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree