Intervention for Acute Myocardial Infarction
William W. O’Neill
The author is a consultant to Medtronic, Inc. Serves on the board of Neovasc, Inc. and has received ongoing research support from ABIOMED, Inc., Edwards Lifesciences, Inc., and Abbott, Inc.
Coronary artery disease in general and acute myocardial infarction (AMI) in particular remain the leading cause of death in Western societies. AMI is also becoming increasingly problematic in South and Central America, India, and China. As a result, it will continue to have enormous worldwide health implications. The understanding of the pathophysiology and development of reperfusion therapy of AMI will rank alongside the discovery of penicillin, isoniazid, and the polio vaccine as one of the major triumphs in medicine of the 20th century. In the early 1970s, when patients were admitted to coronary care units a 19% mortality was expected.1 Currently major angioplasty trials of ST-elevation AMI (STEMI) demonstrate a 1-year mortality of 3.5%.2 This dramatic reduction in mortality has contributed to a 5-year increase in life expectancy in the United States from 1970 to 1990. This chapter will summarize the current thinking on organization and execution of an optimal angioplasty strategy for STEMI.
HISTORICAL OVERVIEW
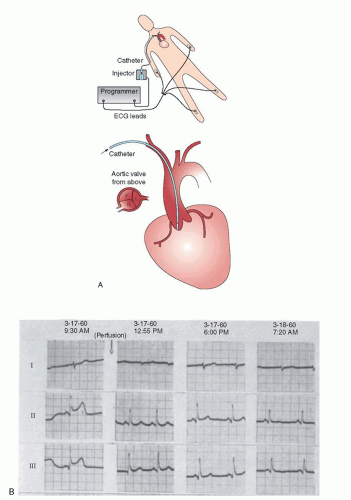
Catheterization-based strategies for management of STEMI date back to a largely overlooked work performed at Jackson Memorial Hospital by Robert Boucek et al. from the University of Miami.3 The work was initiated in 1959. Boucek was aware of the work of Fletcher and Sherry4 with a nonspecific fibrinolytic agent. The Miami investigators performed brachial artery cutdowns, placed nonselective catheters, and infused fibrinolysis in the aortic root of patients with ECG evidence of AMI. They published their work and demonstrated ECG evidence of reperfusion (Figure 30.1). Unfortunately, they were not able to perform selective coronary angiography to actually prove that reperfusion had occurred. Ignatios Voudoukis, a clinical fellow and co-author, explained later that this work was so heavily criticized by the leaders of cardiology in America that Boucek abandoned the work.5 Had Boucek known of the work Sones6 was conducting in Cleveland with selective coronary arteriography, the entire field might have advanced 20 years earlier.
In 1960 when Boucek was pioneering reperfusion, controversy existed over the role of thrombotic occlusion. Pathologic studies found occluding coronary thrombosis in only 50% of autopsy cases. Cardiac enzyme markers of acute MI did not exist, and clinicopathologic differentiation of ST versus non-ST MI had not occurred yet. A major breakthrough came in 19797 when DeWood et al. demonstrated that thrombotic occlusion was extremely prevalent in STEMI, especially if catheterization occurred within 6 hours of symptom onset. Furthermore, total occlusion was much less prevalent among patients catheterized after 6 hours. This suggested that the body’s own fibrinolytic system was reversing some occlusions. With this information, Rentrop8 soon thereafter was able to demonstrate that intracoronary occlusions could be successfully opened with intracoronary streptokinase therapy. Khaja9 next proved that streptokinase was superior to placebo in opening occluded arteries. This was the first randomized trial in the field of reperfusion. Kennedy et al. finally demonstrated that intracoronary streptokinase improved 1-year survival as compared to placebo.10 This group also for the first time linked vessel patency, which they termed “complete” perfusion versus “incomplete perfusion” or no perfusion, to 1-year survival.11 These studies in the early 1980s ushered in the modern era of reperfusion therapy.
At the same time that pharmacologic reperfusion was being introduced, a second parallel cardiovascular intervention also was born: coronary angioplasty therapy. Gruentzig et al. first reported in 1977 that elective angioplasty could be performed in the cath lab on conscious, sedated patients.12 The University of Michigan group was active in the investigation of intracoronary streptokinase, having collaborated with F. Khaja on the original randomized intracoronary streptokinase trial. Based on their experience with streptokinase, they tested it versus coronary angioplasty in the first randomized trial of thrombolytic therapy versus angioplasty (Figure 30.2). This study ushered in the next phase of investigations into reperfusion therapy: mechanical reperfusion.13
Even though intracoronary streptokinase was effective and saved lives, some major drawbacks existed. One of those was the risk of major bleeding, not only at the vascular puncture site, but in remote areas as well. Catastrophic intracranial bleeds occurred in 1% to 2% of cases. These cases were demoralizing especially when this happened in young patients. In addition, angiographic studies revealed severe residual lesions in over 70% of patients in whom successful reperfusion occurred, while in 20% to 30% of cases the therapy was unsuccessful. Coronary angioplasty was an attractive alternative: bleeding risk was lower; intracranial bleeds were unlikely; residual lesions were eliminated; and “complete reperfusion,” now labeled TIMI III (Thrombolysis In Myocardial Infarction) flow, occurred in over 90% of cases.13 However, these theoretical advantages had to be proven in the context of randomized trials.
Unfortunately, the field of mechanical reperfusion lost momentum between 1985 and 1995. During this time
investigation focused on the combination of intravenous thrombolytic therapy with coronary angioplasty. The combination was tested in three trials14, 15, 16 and proven to be dangerous. Bleeding risk was higher, stroke risk was higher, and mortality was increased as compared to intravenous thrombolytic therapy alone.
investigation focused on the combination of intravenous thrombolytic therapy with coronary angioplasty. The combination was tested in three trials14, 15, 16 and proven to be dangerous. Bleeding risk was higher, stroke risk was higher, and mortality was increased as compared to intravenous thrombolytic therapy alone.
Fortunately during this same time balloon angioplasty became further refined, more operators became trained, and large numbers of catheterization laboratories became proficient in elective angioplasty.
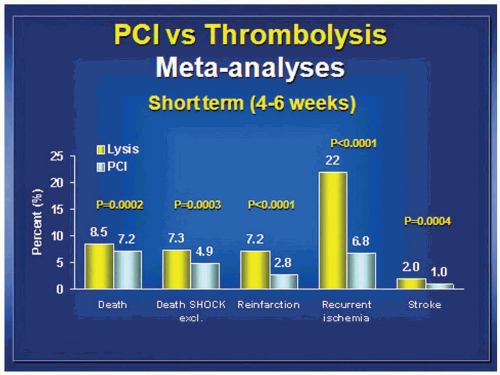
This set the stage for comparative trials of coronary angioplasty versus thrombolytic therapy. Groups in Royal Oak, Michigan17; Rochester, Minnesota18; and Zwolle, Netherlands19 conducted clinical trials which in aggregate demonstrated survival advantage and large reductions in risk of death or reinfarction when angioplasty was used without antecedent thrombolytic therapy. Importantly a 10-fold decrease in the risk of intracranial hemorrhage occurred for angioplasty therapy. The decade between 1995 and 2005 saw 20 further randomized trials conducted, which were summarized by Keeley et al. in a meta-analysis published in 2006.20 In aggregate these trials demonstrated a reduction in mortality (7.4% versus 5.5%, P = 0.0003), reduction in risk of stroke (2% versus 1%, P = 0.0004), reduction in reinfarction (6.8% versus 2.5%, P < 0.0001), and reduction in death or reinfarction (14% versus 8.2%, P < 0.0001) for angioplasty therapy. This summary ushered in the current era of investigation. Mechanical reperfusion had been proven to be safer and superior to thrombolytic therapy. Now the results needed to be optimized. Over the last decade research attention has turned from finding a role for intravenous thrombolytic therapy to optimizing the efficacy, safety, and durability of mechanical reperfusion. The last vestige of hope for a role for intravenous thrombolytic therapy was quashed when Ellis et al. published the Finesse trial21 and ASSENT investigators published the ASSENT IV trial22 (Assessment of the Safety and Efficacy of a New Treatment Strategy for Acute Myocardial Infarction). Ellis found that neither facilitation of PCI with retaplase plus abciximab nor facilitation with abciximab alone improved outcomes as compared to PCI alone. The ASSENT IV investigators found that a strategy of full-dose tenecteplase preceding PCI was associated with an increased number of adverse events than was PCI alone. Keeley et al. recently analyzed 17 randomized trials of PCI alone versus PCI facilitated with thrombolytic therapy20 (Figure 30.3). This meta-analysis demonstrated that mortality, reinfarction, intracranial bleeds, and total stroke are significantly increased when intravenous thrombolytic therapy is combined with PCI. This combination is harmful and should not be routinely applied.
SYSTEMS OF CARE
Angioplasty-mediated reperfusion therapy has enormous efficacy and safety advantages over intravenous thrombolytic therapy. Its major disadvantage, however, is that specialized catheterization facilities and highly trained operators are required to maximize its efficacy. Each hospital has its own obstacles that will take a reperfusion champion to overcome. Bradley23 has found that the major difference between successful and marginal reperfusion programs is that in successful programs a reperfusion champion is able to get emergency departments, ambulance services, cath labs, and the cardiologist to buy into a collaboration for timely administration of therapy. Rapid and accurate identification of STEMI patients in the emergency department, rapid mobilization of cath lab staff and rapid response by the interventional cardiologist are all prerequisites for an effective program. Key performance indicators include a 90-minute door-to-balloon time for >90% of cases, final TIMI III flow in >90% of cases, nonshock mortality in <4% of cases, and a shock mortality in <50% of cardiogenic shock cases.
Angioplasty with No Surgery Onsite
In the United States there are over 5,000 acute care hospitals. Of these, only around 1,000 have coronary bypass surgery and coronary angioplasty programs. For this reason patients with STEMI often present to institutions without angioplasty or bypass available onsite. Wharton pioneered and championed24 the concept of initiating emergency angioplasty in hospitals without onsite surgery. The Primary Angioplasty in MI (PAMI)-No Surgery On site (SOS) registry suggested that angioplasty performed by experienced operators could accomplish similar outcomes to those achieved by established reperfusion centers.25 The Atlantic Cardiovascular
Patient Outcomes Research Team (C-PORT) randomized patients presenting to hospitals in Maryland and Massachusetts to angioplasty therapy onsite versus intravenous accelerated tissue plasminogen activator therapy.26 The C-PORT investigators found that the incidence of death, MI, or stroke was significantly reduced with angioplasty therapy (12.4% versus 19.9%, P = 0.03). These studies demonstrate that institutions with the will to organize programs and obtain cardiology staffing including experienced interventionalists can provide superb reperfusion therapy with angioplasty. It is expected that many states in the United States will allow this approach to expand.
Patient Outcomes Research Team (C-PORT) randomized patients presenting to hospitals in Maryland and Massachusetts to angioplasty therapy onsite versus intravenous accelerated tissue plasminogen activator therapy.26 The C-PORT investigators found that the incidence of death, MI, or stroke was significantly reduced with angioplasty therapy (12.4% versus 19.9%, P = 0.03). These studies demonstrate that institutions with the will to organize programs and obtain cardiology staffing including experienced interventionalists can provide superb reperfusion therapy with angioplasty. It is expected that many states in the United States will allow this approach to expand.
Regional Transfer Centers
In remote, sparsely populated regions angioplasty onsite is not logistically feasible. For such regions, transfer protocols with helicopter or ambulance will still allow for effective mechanical reperfusion therapy. Topol et al.27 first demonstrated the feasibility of helicopter transfer for emergency angioplasty. More recently Henry28 and Ting29 showed that patients presenting as far as 120 miles from a regional center can have outcomes comparable to those of local patients when an organized triage and transfer protocol exists. The viability of this approach was confirmed by the nationwide DANish trial in Acute Myocardial Infarction (DANAMI).30 In this trial patients were randomized to intravenous thrombolytic therapy versus primary angioplasty. Importantly, this was a nationwide trial with 80% of centers not able to perform angioplasty. In those centers patients were identified and transferred for PCI if randomized to that arm. The study was prematurely stopped owing to a mortality advantage for PCI. Thus even with a 120-minute delay for transfer patients, outcomes were improved for the PCI arm. It is crucial to understand that the DANAMI group had an extremely well-organized transfer protocol whereby patients were identified and transferred quickly (door in-door out time of 30 minutes) and referral centers were immediately activated so that doorto-balloon times were minimized at the accepting center. As a follow-up of this study, Denmark has now established primary angioplasty as a nationwide protocol.
Metropolitan Systems
Densely populated urban areas have a different set of issues concerning reperfusion strategy. Traditionally, heavily populated areas have multiple acute care hospitals, all functioning independently with haphazard referral patterns largely based on physician preferences. In the United States half of patients arrive at the hospital by self transport and the other half by ambulance. Traditionally emergency rooms triaged trauma over chest pain. Most hospitals had myriad options for reperfusion. Even in hospitals with onsite PCI programs, reperfusion method could differ depending on time of arrival, physician on call, or weekday versus weekend arrival.
Fortunately, now that PCI has become the dominant reperfusion strategy in the United States, all this has changed. A nationwide imperative to identify, triage, and treat STEMI patients has forced all US acute care hospitals to develop organized STEMI protocols. Public reporting of acute MI core measures now include time for thrombolytic therapy administration and percentage of patients with a door-to-balloon time of <90 minutes.31 Furthermore, the ACC and AHA have fully endorsed organized STEMI protocols as a Class I recommendation.
The National Registry for Myocardial Infarction (NRMI) has documented very encouraging nationwide trends with more patients receiving reperfusion therapy, more hospitals achieving 90-minute door-to-balloon times, and shorter transport times for patients transferred for emergency catheterization.32
Two other strategies will be beneficial for improving outcomes in metropolitan areas: First, citywide reperfusion centers like those in Ottawa33 will reduce reperfusion delays and triage patients to STEMI facilities. In the United States, Los Angeles County and Miami Dade County have well-developed programs whereby ambulance rigs are equipped with systems to transmit ECGs from the field. Furthermore, when a STEMI is identified, patients are taken to the closest STEMI facility. This has resulted in dramatic reductions in door-to-balloon time. Currently it is the goal of Miami Dade County to routinely achieve door-to-balloon times of <60 minutes. Recent data suggest that when door-to-balloon times drop below 60 minutes, a dramatic reduction in mortality occurs.34
As these organized approaches demonstrate value, a second major societal change will occur. Currently there is a delay of over 1 hour for reperfusion when patients arrive by self-transport.35 Since half of US STEMI patients arrive by selftransport, marketing campaigns to persuade people to call for ambulance when a heart attack is suspected will be beneficial for reducing treatment delays. Nonshock mortality rates of under 1% can be achieved by adopting these approaches.
FUNDAMENTAL CONCEPTS OF REPERFUSION
To optimize treatment outcomes, a multipronged approach to the pathophysiology of the clotting system, the coronary lesion, and the ischemic myocardium is required. It is now understood that coronary artery inflammation with macrophage infiltration and destabilization of thin-capped fibroatheroma is the inciting event in vulnerable plaque rupture or erosion.36 This leads to a cascade of platelet activation and aggregation as well as tissue factor-mediated thrombin activation. A platelet-rich, fibrin-laced thrombus quickly develops and totally occludes the affected coronary artery. The local environment of the total occlusion is hostile to
intervention. Clot burden of varying extent occurs, platelet activation is intense, and plaque rupture can cause spontaneous dissections. In some patients even a minor erosion of severe underlying occlusion can totally occlude the vessel. For these reasons, there exists a ceiling of reperfusion for pharmacologic approaches, whereby initial patency rates of <70% occur. Reocclusion rates of 10% to 15% result in a substantial incidence of reinfarction. Thus mechanical opening with pharmacological milieu passivation results in larger numbers of patients with initial successful reperfusion and less reocclusion. Clinical trials have in fact corroborated this superiority. Angioplasty-treated patients are less likely to die or develop shock and less likely to develop reinfarction than those treated with thrombolytic therapy.
intervention. Clot burden of varying extent occurs, platelet activation is intense, and plaque rupture can cause spontaneous dissections. In some patients even a minor erosion of severe underlying occlusion can totally occlude the vessel. For these reasons, there exists a ceiling of reperfusion for pharmacologic approaches, whereby initial patency rates of <70% occur. Reocclusion rates of 10% to 15% result in a substantial incidence of reinfarction. Thus mechanical opening with pharmacological milieu passivation results in larger numbers of patients with initial successful reperfusion and less reocclusion. Clinical trials have in fact corroborated this superiority. Angioplasty-treated patients are less likely to die or develop shock and less likely to develop reinfarction than those treated with thrombolytic therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree