Interrupted Aortic Arch Complex
Richard G. Ohye
Takaaki Suzuki
Eric J. Devaney
Jennifer C. Hirsch-Romano
Edward L. Bove
Interruption of the aortic arch (IAA) is a congenital anomaly characterized by complete discontinuity of blood flow between two portions of the aorta. This malformation may exist as a long-distance physical separation between adjacent segments or in the form of discontinuity between adjacent lumens of vessels that are otherwise connected externally. The latter anomaly is more commonly considered among discussions of coarctation of the aorta and is not discussed further here. IAA occurs uncommonly as an isolated lesion, being frequently associated with a number of complex intracardiac defects. Therefore, the diagnosis and management of IAA complex is best considered in combination with the entire cardiac anomaly. Recent advances in the management of IAA and the associated cardiac defects have resulted in a significant improvement in the overall outcome for patients with this complex anomaly. The approach of a single-stage repair of all coexisting defects simultaneously with the IAA has been shown to be a safe and effective management protocol.
 ANATOMY
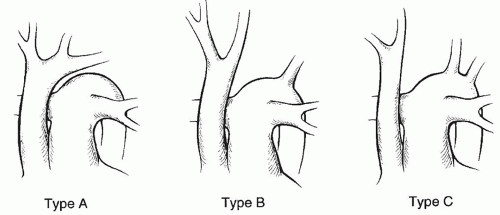
ANATOMYThe aortic arch is that portion of the aorta between the innominate artery and the ductus arteriosus, and interruption of the arch may occur between any of the arch vessels. According to the classification originally described by Celoria and Patton (Fig. 83.1), type A occurs when the aorta is interrupted just distal to the origin of the left subclavian artery, between that vessel and the insertion of the ductus arteriosus itself. In this type, a fibrous cord is frequently found connecting the proximal and distal portions of the arch. The most common variety, type B, occurs between the origins of the left common carotid artery and the left subclavian artery. This type accounts for approximately two-thirds of all cases of IAA. In the rarest form, type C, the interruption occurs between the innominate and left common carotid arteries. This type is seen in only 5% of patients.
Associated cardiovascular anomalies are nearly always present in IAA. The most common condition is that of an isolated ventricular septal defect (VSD). In a multiinstitutional study by the Congenital Heart Surgeons Society (CHSS), which analyzed 250 neonates entered into the study over a 5-year period by 29 participating institutions, isolated VSD was present in 183 patients (73%). Other commonly associated lesions included truncus arteriosus, transposition of the great arteries (TGA) with VSD, and various forms of single ventricle. The frequency of associated anomalies, as found in the CHSS report, is shown in Table 83.1.
The VSD in patients with IAA is frequently of the malalignment type and is commonly associated with posterior deviation of the infundibular (outlet) septum. The displacement of the infundibular septum to the left of the posterior limb of the septal band results in narrowing of the left ventricular outflow tract and the potential for subaortic obstruction. Anomalous origin of the right subclavian artery from the descending aorta frequently occurs with IAA and is associated with a greater prevalence of subaortic obstruction secondary to reduced flow in utero through the left ventricular outflow tract and the aortic valve. Additional levels of left heart obstruction may also occur at the aortic valve leaflets, aortic annulus, mitral valve, and the ascending aorta.
PRESENTATION AND DIAGNOSIS
In the majority of patients, the diagnosis of IAA is first made on the discovery of signs and symptoms of congestive heart failure within the first few days of life. Lower extremity pulses may be poorly palpable or not palpable at all. In some cases, the diagnosis is not suspected until ductal closure occurs. When the ductus remains patent, flow to the lower body remains unobstructed, and the elevated pulmonary vascular resistance normally found in the newborn delays the expected increase in pulmonary blood flow through the VSD. This combination effectively delays the development of heart failure. Abrupt ductal closure, however, results in profound acidosis, cardiovascular collapse, and shock as lower body perfusion is severely reduced. Resuscitation with an infusion of prostaglandin E1 to maintain ductal patency should be established when the diagnosis is made. If shock has occurred and there is associated renal and hepatic dysfunction, administration of dopamine is generally used as well. A period of a few days may be necessary to allow recovery of end-organ function before operative repair is performed. During this time, careful control of ventilation is needed to avoid hyperventilation, which serves to increase pulmonary blood flow and may worsen systemic perfusion further. In addition, treatment of associated conditions, including sepsis, necrotizing enterocolitis, and coagulation abnormalities, must be performed. Because 22q11 monoallelic microdeletion is a commonly associated condition in patients with IAA, occurring in 27% of patients in the CHSS report, careful control of calcium balance will often be necessary. All transfused blood should be radiated to avoid graft-versus-host disease until the diagnosis of 22q11 monoallelic microdeletion is definitively excluded.
The diagnosis of IAA can be accurately made from two-dimensional Doppler/echocardiographic studies. Cardiac catheterization with aortic angiography is rarely needed to define the anatomy. The exact site of the interruption in addition to the location of the branch vessels and the distance between interrupted segments should be determined. The presence of an anomalous origin of the right subclavian artery may be more difficult to diagnose by echocardiography but would not significantly alter the operative approach. In addition to the anatomy of the aortic arch, it is important to define the intracardiac anatomy in anticipation of a complete one-stage repair. The location
and boundaries of the VSD, particularly in relation to the left ventricular outflow tract, aortic valve, and pulmonary valve, must be accurately seen. The majority of VSDs occur in the outlet portion of the septum, but other locations as well as additional defects must be sought. Although accurate measurement of a gradient across the left ventricular outflow tract is not possible in the presence of a nonrestrictive VSD and patent ductus arteriosus, some guidelines are helpful in predicting those patients who are likely to develop important left ventricular outflow tract obstruction after repair. When the measured ratio of the smallest diameter of the left ventricular outflow tract normalized to the diameter of the descending thoracic aorta at the level of the diaphragm is 1.0 as measured by echocardiography in diastole or is 0.6 when measured in systole, we have found that the risk of subaortic obstruction after closure of the VSD is high and that efforts to resect or incise that portion of the infundibular septum that is deviated posteriorly beneath the aortic annulus are beneficial in avoiding or reducing postoperative obstruction. Other groups have suggested other preoperative echocardiographic measurements, including cross-sectional area of the left ventricular outflow tract indexed to body surface area, subaortic diameter index, and subaortic diameter Z score. However, the optimal parameter, which consistently displays a high degree of sensitivity and specificity, remains elusive.
and boundaries of the VSD, particularly in relation to the left ventricular outflow tract, aortic valve, and pulmonary valve, must be accurately seen. The majority of VSDs occur in the outlet portion of the septum, but other locations as well as additional defects must be sought. Although accurate measurement of a gradient across the left ventricular outflow tract is not possible in the presence of a nonrestrictive VSD and patent ductus arteriosus, some guidelines are helpful in predicting those patients who are likely to develop important left ventricular outflow tract obstruction after repair. When the measured ratio of the smallest diameter of the left ventricular outflow tract normalized to the diameter of the descending thoracic aorta at the level of the diaphragm is 1.0 as measured by echocardiography in diastole or is 0.6 when measured in systole, we have found that the risk of subaortic obstruction after closure of the VSD is high and that efforts to resect or incise that portion of the infundibular septum that is deviated posteriorly beneath the aortic annulus are beneficial in avoiding or reducing postoperative obstruction. Other groups have suggested other preoperative echocardiographic measurements, including cross-sectional area of the left ventricular outflow tract indexed to body surface area, subaortic diameter index, and subaortic diameter Z score. However, the optimal parameter, which consistently displays a high degree of sensitivity and specificity, remains elusive.
Table 83.1 Associated Cardiovascular Conditions in Neonates with Interrupted Aortic Arch | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
OPERATIVE MANAGEMENT OF INTERRUPTED AORTIC ARCH WITH ISOLATED VENTRICULAR SEPTAL DEFECT
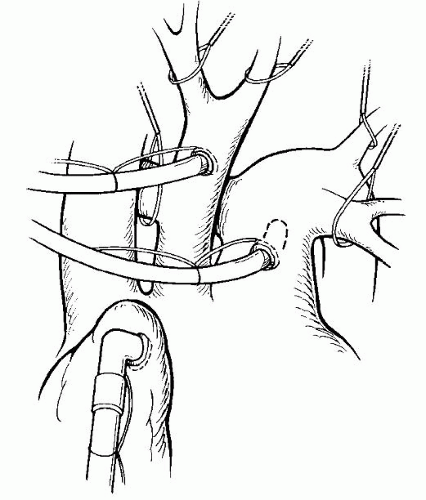
The preferred operative management of a newborn with IAA and isolated VSD is a single-stage repair performed through a midline sternotomy approach. Nasopharyngeal and rectal temperature probes are placed. Arterial monitoring is achieved by the placement of a catheter in the umbilical or femoral artery, which has usually been accomplished in the intensive care unit before surgery. Additional noninvasive arterial monitoring should be done with a blood pressure cuff on the right arm to allow measurement of blood pressures above and below the repair site. If the right radial artery has already been cannulated, the cuff is placed on the leg. Venous access is accomplished through an umbilical or femoral venous catheter; internal jugular access is avoided in babies smaller than approximately 5 kg because of the increased risk of superior vena caval thrombosis. A midline sternotomy incision is made, and the presence or absence of thymic tissue is noted. If the thymus is present, it is totally or partially resected for exposure. Heparin is administered at this time, and the pericardium is opened. Purse-string sutures for cannulation are placed in the right atrial appendage, the ascending aorta at the base of the innominate artery, and the main pulmonary artery (Fig. 83.2). Alternatively, bicaval venous cannulation may be established at this time. A tourniquet is placed around the right pulmonary artery before bypass, and this vessel may be occluded at this time if significant hypotension exists. A Y-adapter is placed in the arterial line of the pump to allow cannulation of both the ascending aorta and pulmonary artery. Once bypass is initiated, a tourniquet is placed around the left pulmonary artery and is engaged (as well as the right if not already done) to direct all pump flow through the ductus and to the lower body. Alternatively, a tourniquet may be engaged around the ductus itself to exclude pump flow into the pulmonary circuit. This method of cannulation allows for even cooling of the upper and lower body in preparation for circulatory arrest. Bypass is initiated at a blood temperature of approximately 30°C, and progressive cooling to a nasopharyngeal temperature of 18°C or less is accomplished. Ice bags are placed around the head. Systemic vasodilatation with phentolamine (0.1 mg/kg) is given to facilitate even cooling between the nasopharyngeal
and rectal temperatures. A minimal cooling period of 20 minutes is allowed, regardless of when the desired temperatures are reached.
and rectal temperatures. A minimal cooling period of 20 minutes is allowed, regardless of when the desired temperatures are reached.
During the interval required for cooling, the ascending and descending aorta and the brachiocephalic vessels are mobilized widely. Sufficient intercostal arteries are divided to permit a tension-free anastomosis. After appropriate cooling, the circulation is arrested, and the arch vessels are snared. Blood cardioplegia is delivered through a separate cannula after placement of a cross-clamp or through the arterial cannulation site to induce arrest. The ductus arteriosus is ligated on the pulmonary artery side and excised distally, with care being taken to remove all ductal tissues on the descending aorta. A vascular clamp on the descending aorta facilitates exposure and avoids the need to place excess tension with forceps on the vessel itself. When there is an anomalous right subclavian artery, ligation and division are usually required to permit an anastomosis without tension or compression of the trachea. In the case of a type B IAA, the descending aorta is sutured to an opening beginning at the base of the left common carotid artery, which is extended proximally along the underside of the ascending aorta (Fig. 83.3). Proper alignment is important, and care must be taken to avoid spiraling this incision. The anastomosis is performed with a continuous technique using 6-0 or 7-0 absorbable or nonabsorbable monofilament suture. In cases of type A IAA, the transverse aortic arch may be hypoplastic, as it is frequently seen with coarctation of the aorta. The anastomosis should be spatulated to enlarge the transverse arch using the distal aorta, bringing the suture line proximally to the level of the innominate artery to ensure that there is no residual narrowing.
The total elapsed circulatory arrest time at this point of the procedure is generally no more than 12 to 15 minutes, allowing ample time for VSD closure through an incision in the right atrium. Alternatively, bicaval cannulation can be utilized, rather than single atrial access. If this approach is employed, cardiopulmonary bypass may be resumed at this point for the VSD closure to minimize circulatory arrest time. Occasionally, when there is deficiency or absence of the infundibular septum and overriding of the VSD by the pulmonary valve, optimal exposure is best achieved through an incision in the main pulmonary artery. Transatrial exposure of the defect is usually accomplished easily with traction sutures placed on the anterior and septal leaflets of the tricuspid valve, and an appropriately trimmed patch of polytetrafluoroethylene material is placed with a continuous 6-0 polypropylene suture. When there is coexisting posterior deviation of the infundibular septum (Fig. 83.4), it has been our routine practice to perform a wedge resection of the septum through the VSD (Figs. 83.5, 83.6, 83.7). On occasion, an incision in the septum alone is performed, which is usually sufficient to enlarge the left ventricular outflow tract. The atriotomy is closed, and the cannulae are reinserted for cardiopulmonary bypass if the VSD was closed under circulatory arrest, with air being evacuated through a generous needle hole in the ascending aorta. Rewarming is accomplished, during which time epicardial pacing wires are placed on the right atrium and ventricle. After bypass is discontinued, one or two additional catheters are placed in the right atrium through the cannulation purse string. Left atrial pressure monitoring lines may also be placed. Routine sternal closure over a mediastinal drain is performed; occasionally, sternal closure is delayed if there is hemodynamic compromise or significant edema.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree