After reading this chapter you will be able to: Beginning in the 1970s,1 the term normal ranges was slowly replaced with more appropriate terms such as reference ranges, biologic reference intervals, and expected value.2 This change in terminology acknowledged that what we consider normal must take into account variations related to age, gender, race, and ethnicity, which change over time as the demographic composition of society changes. A reference range sets the boundaries for any analyte (e.g., electrolyte, blood cell, protein, enzyme) that would likely be encountered in healthy subjects. This range would encompass the variability reflected in the larger, presumably healthy population. Reference ranges vary from laboratory to laboratory for various reasons, including differences in measurement techniques, the populations of healthy individuals used to establish the reference intervals, or analytic imprecision when the intervals were constructed. Most differences in reference ranges are relatively small, with reasonably close agreement between most laboratories.2 The reference ranges and critical values given in this chapter are from a single institution, and they serve as representative examples. RTs must become familiar with the reference ranges used at their institutions. In this chapter, critical values are listed along with common pathophysiologic states with which they commonly occur. Not all clinical analytes have an associated critical value. For some tests, there is no general agreement on what a critical value would be. Others have only a one-sided value that exists below or above a critical threshold; this is true particularly for substances that do not normally appear in the blood. For example, certain enzymes and proteins are released only after extensive cellular damage following injury (see later section on enzyme tests). Under normal circumstances, these proteins or enzymes may be virtually undetectable in the serum or plasma. The complete blood count (CBC) provides a detailed description of the number of circulating white blood cells (WBCs), called leukocytes; red blood cells (RBCs), called erythrocytes; and platelets, called thrombocytes. The WBC count is made up of five different types of cells and is reported under the differential. RBCs are evaluated for size and hemoglobin content. The platelets are evaluated for number present. Table 16-1 lists the normal CBC results for adults. TABLE 16-1 Reference Range Values for Complete Blood Count in an Adult Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011. The differential of the WBC count determines the exact number of each type of WBC present in the circulating blood (Table 16-2). Most circulating WBCs are either neutrophils or lymphocytes. Because leukocytosis usually results from only one of the five cell types responding to a problem, significant elevation of the WBC count (>15 × 103/mcl) occurs only when either neutrophils or lymphocytes are responding to an abnormality. Because basophils, eosinophils, and monocytes make up such a small proportion of the circulating WBCs, they are not likely to cause a major increase in the WBC count when responding to disease. TABLE 16-2 Reference Range Values for White Blood Cell Count Differential and Common Causes for Abnormalities Values for reference ranges and critical test results are from the University of California San Francisco Moffit-Long Hospital and San Francisco General Hospital. http://pathology.ucsf.edu/labmanual/mftlng-mtzn/test/test-index.html and http://pathology.ucsf.edu/sfghlab/test/ReferenceRanges.html. Accessed January 1, 2011.
Interpreting Clinical and Laboratory Data
 Describe what a critical value is, and state its importance in clinical practice.
Describe what a critical value is, and state its importance in clinical practice.
 Define the following terms related to clinical laboratory tests: leukocytosis, leukopenia, anemia, polycythemia, and thrombocytopenia.
Define the following terms related to clinical laboratory tests: leukocytosis, leukopenia, anemia, polycythemia, and thrombocytopenia.
 Identify which electrolyte disturbances interfere with normal respiratory function.
Identify which electrolyte disturbances interfere with normal respiratory function.
 Describe clinical tests used to identify cardiac stress and myocardial infarction.
Describe clinical tests used to identify cardiac stress and myocardial infarction.
 Identify the three main tests used to diagnose coagulation disorders.
Identify the three main tests used to diagnose coagulation disorders.
 Describe how the sputum Gram stain and culture are used to diagnose pulmonary infections.
Describe how the sputum Gram stain and culture are used to diagnose pulmonary infections.
Interpreting Clinical Laboratory Tests
Introduction to Laboratory Medicine
Reference Range
Critical Test Value
Complete Blood Count
Test
Reference Range
Red blood cell count
Men
4.4-5.9 × 106/mcl
Women
3.8-5.2 × 106/mcl
Hemoglobin
Men
13.3-17.7 g/dl
Women
11.7-15.7 g/dl
Hematocrit
Men
40%-52%
Women
35%-47%
White blood cell count
3.9-11.7 × 103/mcL
White blood cell differential
Segmented neutrophils
40%-75%
Bands
0%-6%
Eosinophils
0%-6%
Basophils
0%-1%
Lymphocytes
20%-45%
Monocytes
2%-10%
Platelet count
150-400 × 103/mcL
White Blood Cell Count
White Blood Cell Count Differential
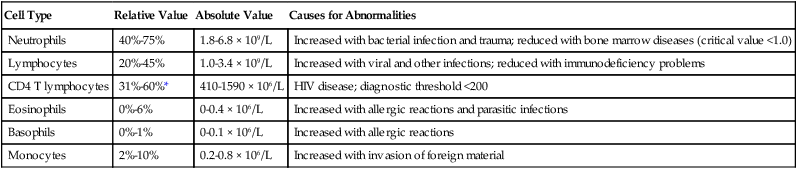
Cell Type
Relative Value
Absolute Value
Causes for Abnormalities
Neutrophils
40%-75%
1.8-6.8 × 109/L
Increased with bacterial infection and trauma; reduced with bone marrow diseases (critical value <1.0)
Lymphocytes
20%-45%
1.0-3.4 × 109/L
Increased with viral and other infections; reduced with immunodeficiency problems
CD4 T lymphocytes
31%-60%*
410-1590 × 106/L
HIV disease; diagnostic threshold <200
Eosinophils
0%-6%
0-0.4 × 106/L
Increased with allergic reactions and parasitic infections
Basophils
0%-1%
0-0.1 × 106/L
Increased with allergic reactions
Monocytes
2%-10%
0.2-0.8 × 106/L
Increased with invasion of foreign material
Interpreting Clinical and Laboratory Data



