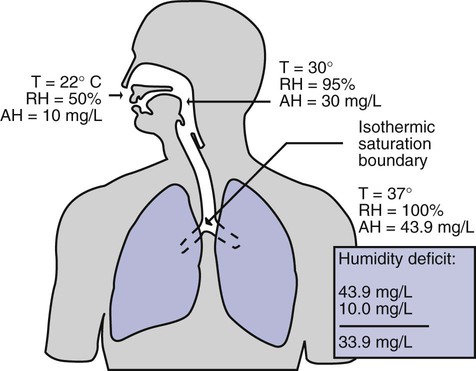
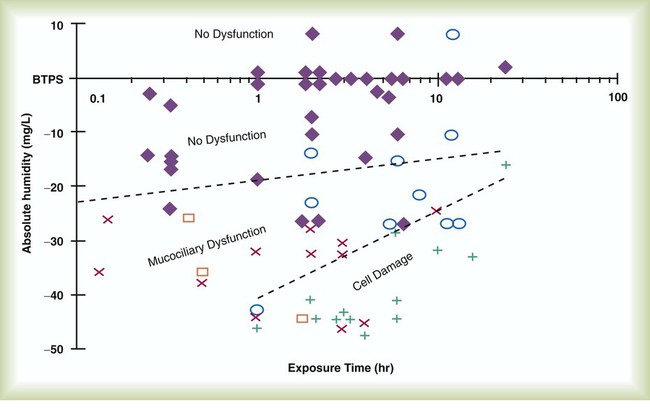
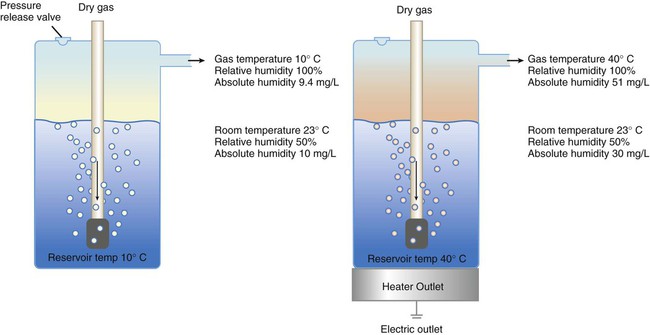
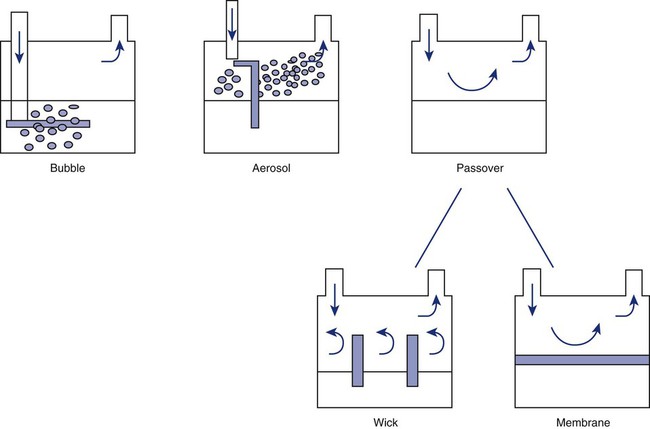
After reading this chapter you will be able to: Vapors and mists have been used for thousands of years to treat respiratory disease. Modern respiratory care still uses these treatments at the bedside, in the form of water vapor (humidity) and bland water aerosols. Concepts of absolute and relative humidity are essential for understanding humidity therapy; these concepts are covered in Chapter 6. This chapter reviews the principles, methods, equipment, and procedures for using these concepts appropriately. Heat and moisture exchange is a primary function of the upper respiratory tract, mainly the nose.1 The nose heats and humidifies gas on inspiration and cools and reclaims water from gas that is exhaled. The nasal mucosal lining is kept moist by secretions from mucous glands, goblet cells, transudation of fluid through cell walls, and condensation of exhaled humidity. The nasal mucosa is very vascular, actively regulating temperature changes in the nose and serving as an active element in promoting effective heat transfer. Similarly, the mucosa lining the sinuses, trachea, and bronchi aid in heating and humidifying inspired gases. The mouth is less effective at heat and moisture exchange than the nose because of the low ratio of gas volume to moist and warm surface area and the less vascular squamous epithelium lining the oropharynx and hypopharynx. When a person inhales through the mouth at normal room temperature, pharyngeal temperatures are approximately 3° C less than when the person breathes through the nose, with 20% less relative humidity. During exhalation, the relative humidity of expired gas varies little between mouth breathing and nose breathing, but the mouth is much less efficient in reclaiming heat and water.2 As inspired gas moves into the lungs, it achieves BTPS conditions (body temperature, 37° C; barometric pressure; saturated with water vapor [100% relative humidity at 37° C]) (Figure 35-1). This point, normally approximately 5 cm below the carina, is called the isothermic saturation boundary (ISB).3 Above the ISB, temperature and humidity decrease during inspiration and increase during exhalation. Below the ISB, temperature and relative humidity remain constant (BTPS). The primary goal of humidification is to maintain normal physiologic conditions in the lower airways. Proper levels of heat and humidity help ensure normal function of the mucociliary transport system. Humidity therapy is also used to treat abnormal conditions. Box 35-1 summarizes the primary and secondary indications for humidity therapy. The hazard of breathing dry gas is even greater when the normal heat and water exchange capabilities of the upper airway are lost or bypassed, as occurs with endotracheal intubation.4 Breathing dry gas through an endotracheal tube can cause damage to tracheal epithelium within minutes. However, as long as the inspired humidity is at least 60% of BTPS conditions, no injury occurs in normal lungs.5,6 Prolonged breathing of improperly conditioned gases through a tracheal airway can result in hypothermia (reduced body temperature), inspissation of airway secretions, mucociliary dysfunction, destruction of airway epithelium, and atelectasis.7 Box 35-2 summarizes the signs and symptoms associated with breathing cold, dry gases. Figure 35-2 illustrates the level of dysfunction in the airway caused by changes in absolute humidity below BTPS and over hours of exposure. A reduction of 20 mg/L below BTPS (44 mg/L) is less than 60% relative humidity at BTPS. The amount of heat and humidity that a patient needs depends on the site of gas delivery (e.g., nose or mouth, hypopharynx, trachea). Table 35-1 summarizes the recommended levels based on current standards.8 TABLE 35-1 Recommended Heat and Humidity Levels From Chatburn R, Primiano F: A rational basis for humidity therapy. Respir Care 32:249, 1987. Warmed, humidified gases are used to prevent or treat various abnormal conditions. For treatment of a patient with hypothermia, heating and humidifying the inspired gas is one of several techniques used to raise core temperatures back to normal.9,10 Heated humidification is also used to prevent intraoperative hypothermia.11 Of possibly greater clinical significance, warming and humidifying the inspired gas can help alleviate bronchospasm in patients who develop airway narrowing after exercise or when they breathe cold air. Although the cause of this condition is not known for certain, the primary stimulus is probably a combination of airway cooling and drying, which leads to hypertonicity of airway lining fluid and the release of chemical mediators.12 Patients may reduce the incidence of cold air–induced bronchospasm by simply wearing a scarf over the nose and mouth when outside in cold weather; the scarf serves as a crude passive heat and moisture exchanger (HME). A humidifier is a device that adds molecular water to gas. This process occurs by evaporation of water from a surface (see Chapter 6), whether the water is in a reservoir, a wick, or a sphere of water in suspension (aerosol). The following four variables or principles affect the quality of performance of a humidifier: (1) temperature, (2) surface area, (3) time of contact, and (4) thermal mass. These factors are exploited to various degrees in the design of humidification devices (Box 35-3). Figure 35-3 shows this concept, where, owing to evaporative cooling, the unheated humidifier on the left is operating at 10° C. Although the humidifier fully saturates the gas, the low operating temperature limits total water vapor capacity to approximately 9.4 mg/L water vapor, equivalent to approximately 21% of body humidity. Simply heating the humidifier to 40° C (see Figure 35-3, right) increases its output to 51 mg/L, which is sufficient to meet BTPS conditions. Humidifiers are either active (actively adding heat or water or both to the device-patient interface) or passive (recycling exhaled heat and humidity from the patient). Active humidifiers typically include (1) bubble humidifiers, (2) passover humidifiers, (3) nebulizers of bland aerosols, and (4) vaporizers. Passive humidifiers refer to typical heat and moisture exchangers (HMEs). Specifications covering the design and performance requirements for medical humidifiers are established by the American Society for Testing and Materials (ASTM).13 A bubble humidifier breaks (diffuses) an underwater gas stream into small bubbles (Figure 35-4). Use of a foam or mesh diffuser produces smaller bubbles than an open lumen, allowing greater surface area for gas/water interaction. Unheated bubble humidifiers are commonly used with oxygen (O2) delivery systems (see Chapter 38) to raise the water vapor content of the gas to ambient levels. As indicated in Table 35-2, unheated bubble humidifiers can provide absolute humidity levels between approximately 15 mg/L and 20 mg/L.14–16 At room temperature, 10 mg/L absolute humidity corresponds to approximately 80% relative humidity but only approximately 25% body humidity (see Chapter 6). As gas flow increases, these devices become less efficient as the reservoir cools and contact time is reduced, limiting their effectiveness at flow rates greater than 10 L/min. Heating the reservoirs of these units can increase humidity content, but this is not recommended because the resulting condensate tends to obstruct the small bore delivery tubing to which these units connect. TABLE 35-2 Absolute Humidity (mg/L) Provided by Unheated Bubble Humidifiers Modified from Darin J, Broadwell J, MacDonell R: An evaluation of water-vapor output from four brands of unheated, prefilled bubble humidifiers. Respir Care 27:41, 1982. To warn of flow-path obstruction and to prevent bursting of the humidifier bottle, bubble humidifiers incorporate a simple pressure-relief valve, or pop-off. Typically, the pop-off is either a gravity or spring-loaded valve that releases pressures greater than 2 psi. Humidifier pop-offs should provide both an audible and a visible alarm and should automatically resume normal position when pressures return to normal.13 The pop-off also can be used to test an O2 delivery system for leaks. If the system is obstructed at or near the patient interface and the pop-off sounds, the system is leak-free; failure of the pop-off to sound may indicate a leak (or a faulty pop-off valve). At high flow rates, bubble humidifiers can produce aerosols. Although invisible to the naked eye, these water droplet suspensions can transmit pathogenic bacteria from the humidifier reservoir to the patient.17 Because any device that generates an aerosol poses a high risk of spreading infection, strict infection control procedures must be followed when using these systems (see Chapter 4). Passover humidifiers direct gas over a surface containing water. There are three common types of passover humidifiers: (1) simple reservoir type, (2) wick type, and (3) membrane type (see Figure 35-4). A membrane-type humidifier separates the water from the gas stream by means of a hydrophobic membrane (see Figure 35-4). Water vapor molecules can easily pass through this membrane, but liquid water (and pathogens) cannot. As with a wick humidifier, bubbling does not occur. If a membrane-type humidifier were to be inspected while it was in use, no liquid water would be seen in the humidifier chamber. Compared with bubble humidifiers, passover humidifiers offer several advantages.17,18 First, in contrast to bubble devices, passover humidifiers can maintain saturation at high flow rates. Second, they add little or no flow resistance to spontaneous breathing circuits. Third, they do not generate any aerosols, and they pose a minimal risk for spreading infection. Traditionally, use of HMEs has been limited to providing humidification to patients receiving invasive ventilatory support via endotracheal or tracheostomy tubes. More recently, HMEs have been used successfully in meeting the short-term humidification needs of spontaneously breathing patients with tracheostomy tubes.19 Kapadia20 reviewed airway accidents in the intensive care unit for a 4-year period and noted an increasing trend in the incidence of blocked tracheal tubes, which was associated with an increased duration of HME filter use. More recent evidence supports long-term use of HMEs for spontaneously breathing patients.21 Hygroscopic condenser humidifiers provide higher efficiency by (1) using a condensing element of low thermal conductivity (e.g., paper, wool, foam) and (2) impregnating this material with a hygroscopic salt (calcium or lithium chloride). By using an element with low thermal conductivity, hygroscopic condenser humidifiers can retain more heat than simple condenser systems. In addition, the hygroscopic salt helps capture extra moisture from the exhaled gas. During exhalation, some water vapor condenses on the cool condenser element, whereas other water molecules bind directly to the hygroscopic salt. During inspiration, the lower water vapor pressure in the inspired gas liberates water molecules directly from the hygroscopic salt, without cooling. Figure 35-5 depicts the overall process of humidification with a hygroscopic condenser humidifier, showing the changes in temperature and the relative and absolute humidity occurring during the cycle of breathing. As shown, these devices typically achieve approximately 70% efficiency (40 mg/L exhaled, 27 mg/L returned). Hydrophobic condenser humidifiers use a water-repellent element with a large surface area and low thermal conductivity (Figure 35-6). During exhalation, the condenser temperature increases to approximately 25° C because of conduction and latent heat of condensation. On inspiration, cool gas and evaporation cools the condenser down to 10° C. This large temperature change results in the conservation of more water to be used in humidifying the next breath. The efficiency of these devices is comparable to hygroscopic condenser humidifiers (approximately 70%). However, some hydrophobic humidifiers that provide bacterial filtration may reduce the risk of pneumonia but be unsuitable for patients with limited respiratory reserve or who are prone to airway blockage because they may increase artificial airway occlusion.22,23 Design and performance standards for HMEs are set by the International Organization for Standardization (ISO).24 The ideal HME should operate at 70% efficiency or better (providing at least 30 mg/L water vapor); use standard connections; have a low compliance; and add minimal weight, dead space, and flow resistance to a breathing circuit.25 According to Lellouche and colleagues,26 HME performance varies from brand to brand, and only 37.5% of 32 HMEs tested in the study performed well. Table 35-3 compares performance of several commercially available HMEs according to their moisture output, flow resistance, and dead space.26 TABLE 35-3 Comparison of 25 Heat and Moisture Exchangers AH, Absolute humidity; NA, not available. Modified from Lellouche F, Taille S, Lefrancois F, et al: Humidification performance of 48 passive airway humidifiers: comparison with manufacturer data. Chest 135:276, 2009. As shown in Table 35-3, the moisture output of HMEs tends to decrease at high volumes and rates of breathing. In addition, high inspiratory flows and high FiO2 levels can decrease HME efficiency.25
Humidity and Bland Aerosol Therapy
 Describe how airway heat and moisture exchange normally occurs.
Describe how airway heat and moisture exchange normally occurs.
 State the effect dry gases have on the respiratory tract.
State the effect dry gases have on the respiratory tract.
 State when to humidify and warm inspired gas.
State when to humidify and warm inspired gas.
 Describe how various types of humidifiers work.
Describe how various types of humidifiers work.
 Describe how to enhance humidifier performance.
Describe how to enhance humidifier performance.
 State how to select and use humidifier heating and feed systems safely.
State how to select and use humidifier heating and feed systems safely.
 Identify the indications, contraindications, and hazards that pertain to humidification during mechanical ventilation.
Identify the indications, contraindications, and hazards that pertain to humidification during mechanical ventilation.
 Describe how to monitor patients receiving humidity therapy.
Describe how to monitor patients receiving humidity therapy.
 Describe how to identify and resolve common problems with humidification systems.
Describe how to identify and resolve common problems with humidification systems.
 State when to apply bland aerosol therapy.
State when to apply bland aerosol therapy.
 Describe how large volume aerosol generators work.
Describe how large volume aerosol generators work.
 Identify the delivery systems used for bland aerosol therapy.
Identify the delivery systems used for bland aerosol therapy.
 Describe how to identify and resolve common problems with aerosol delivery systems.
Describe how to identify and resolve common problems with aerosol delivery systems.
 Describe how to perform sputum induction.
Describe how to perform sputum induction.
 State how to select the appropriate therapy to condition a patient’s inspired gas.
State how to select the appropriate therapy to condition a patient’s inspired gas.
Humidity Therapy
Physiologic Control of Heat and Moisture Exchange
Indications for Humidification and Warming of Inspired Gases
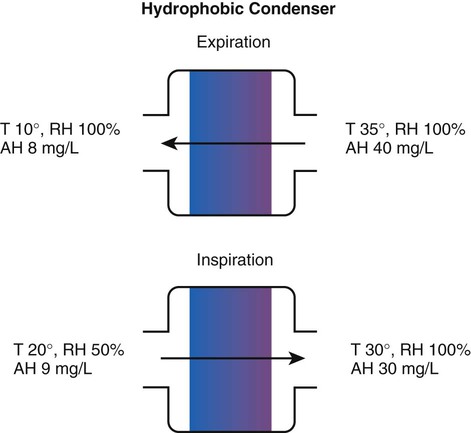
Delivery Site
Temperature Range (° C)
Relative Humidity (%)
Absolute Humidity (mg/L)
Nose/mouth
20-22
50
10
Hypopharynx
29-32
95
28-34
Trachea
32-35
100
36-40
Equipment
Physical Principles Governing Humidifier Function
Temperature
Types of Humidifiers
Active Humidifiers
Bubble
L/min
Aquapak 301 (Hudson RCI, Corp, Dunham, NC)
Traveral 500 (Baxter-Travenol, Corp, Deerfield, IL)
2
17.6
20.4
4
17.7
19.5
6
16.9
16.2
8
14.9
15.7
Passover
Heat and Moisture Exchangers
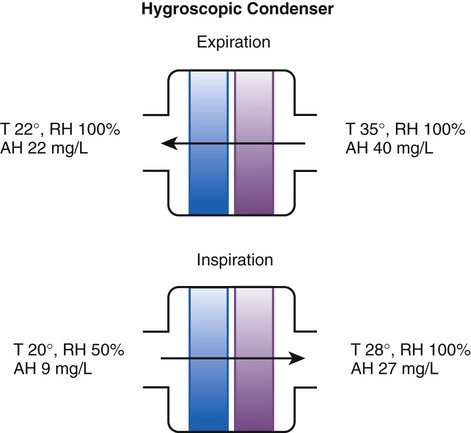
Device
Manufacturer
Measured AH (mg H2O/L)
AH/ml of Dead Space
Measured Resistance at 60 L/min cm H2O
Hygrovent
Peters
31.9 ± 0.6
0.34
1.8
Hygrobac
Mallinckrodt
31.7 ± 0.7
0.33
2.1
Hygrovent S
Peters
31.7 ± 0.5
0.58
2.8
Hygrobac S
Mallinckrodt
31.2 ± 0.2
0.69
2.3
9000/100
Allégiance
31.2 ± 1.4
0.35
2.7
Servo Humidifier 172
Siemens
30.9 ± 0.3
0.56
NA
Humid Vent Filter
Hudson
30.8 ± 0.3
0.88
2.3
Hygroster
Mallinckrodt
30.7 ± 0.6
0.32
2.3
Humid Vent 2
Hudson
29.7 ± 0.4
1.03
NA
Servo Humidifier 162
Siemens
29.7 ± 0.8
0.78
NA
Humid Vent 2S
Hudson
29.2 ± 0.4
1.01
NA
9040/01
Allégiance
28.6 ± 1.1
0.61
2.4
9000/01
Allégiance
28.5 ± 0.8
0.32
3.9
BB100E
Pall
27.2 ± 0.7
0.32
1.4
BB100
Pall
26.8 ± 0.5
0.30
2.0
Stérivent
Mallinckrodt
23.8 ± 0.9
0.26
1.9
Iso Gard Hepa Light
Hudson
23.6 ± 0.3
0.47
2.4
Stérivent S
Mallinckrodt
22.2 ± 0.2
0.36
1.7
BB25
Pall
19.6 ± 1.4
0.56
2.6
BB2000AP
Pall
18.9 ± 0.4
0.54
3.1
Stérivent Mini
Mallinckrodt
16.6 ± 1.0
0.47
2.2
4444/66
Allégiance
16.4 ± 0.6
0.35
3.4
4000/01
Allégiance
15.1 ± 0.9
0.40
2.2
Barrierbac S
Mallinckrodt
13.2 ± 0.2
0.38
2.1
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree