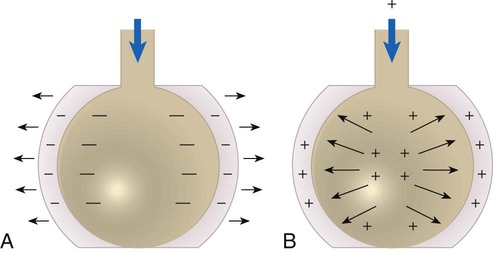
After reading this chapter you will be able to: Pulmonary complications are common serious problems seen in patients who have undergone thoracic or abdominal surgery.1,2 Such complications include atelectasis (alveolar collapse), pneumonia, and acute respiratory failure. These respiratory problems can be minimized or avoided if proper respiratory care is implemented during the perioperative period. The most common form of therapy used in high-risk patients is lung expansion therapy. Various lung expansion therapies can be effective in preventing or correcting atelectasis in selected patients.1 However, the precise method to apply in a given situation is not always clear because no advantage of any one method has been established. The most efficient use of resources is a primary concern with any plan to apply lung expansion therapy. Compression atelectasis results when the forces within the chest wall and lung—specifically, the pleural pressure—are exceeded by the transmural pressure, which is what distends and maintains the alveoli in an open state.2–4 Compression atelectasis is primarily caused by persistent use of small tidal volumes by the patient. This situation is common when general anesthesia is given, with the use of sedatives and bed rest, and when deep breathing is painful, as when broken ribs are present or surgery has been performed on the upper abdominal region. Weakening or impairment of the diaphragm can also contribute to compression atelectasis. Compression atelectasis results when the patient does not periodically take a deep breath and expand the lungs fully. It is a common cause of atelectasis in hospitalized patients. It may occur in combination with gas absorption atelectasis in a patient with excessive airway secretions who breathes with small tidal volumes for a prolonged period. Atelectasis can occur in any patient who cannot or does not take deep breaths periodically and in patients who are restricted to bed rest for any reason.5 Patients who have difficulty taking deep breaths without assistance include patients with significant obesity, patients with neuromuscular disorders or who are under heavy sedation, and patients who have undergone upper abdominal or thoracic surgery. Diaphragmatic position and function is the major contributor to the onset of atelectasis. In an anesthetized patient, there is a cephalad (toward the head) shift of the diaphragm. For patients who are supine, the lower, dependent portion of the diaphragm performs the most movement. The opposite occurs in patients who are paralyzed—the upper portion of the diaphragm is involved in movement.3,4 Patients undergoing lower abdominal surgery are at less risk for atelectasis than patients undergoing upper abdominal or thoracic surgery, but they still may be at significant risk. Patients with spinal cord injury are prone to respiratory complications, the most common of which is atelectasis. Bedridden patients, such as patients recovering from major trauma, are particularly predisposed to developing atelectasis secondary to lack of mobility. Atelectasis is one of the most important determinants of hypoxemia after abdominal surgery and may account for 24% of deaths within 6 days of surgery.6 It is clinically prudent to consider atelectasis in every assessment of postoperative patients. Impairment of the function of pulmonary surfactant can also have an impact on the development of atelectasis. Surfactants decrease the surface tension of the walls of the alveoli. When there is deterioration of the function of this vital protein, the relative increase in surface tension can cause the walls of the alveoli to collapse.4 With all else being constant, the greater the Pl gradient, the more that the alveoli expand. As depicted in Figure 39-1, the Pl gradient can be increased by either (1) decreasing the surrounding Ppl (see Figure 39-1, A) or (2) increasing the Palv (see Figure 39-1, B). A spontaneous deep inspiration increases the Pl gradient by decreasing the Ppl. The application of positive pressure to the lungs increases the Pl gradient by increasing the pressure inside the lung. The goal of any lung expansion therapy should be to implement a plan that provides an effective strategy in the most efficient manner. Staff time and equipment are the two major issues related to efficiency. For a patient with minimal risk of postoperative atelectasis, deep breathing exercises, frequent repositioning, and early ambulation are usually effective and can be done with minimal coaching and time from clinicians and without equipment.4 For a patient at high risk for atelectasis (e.g., a patient undergoing upper abdominal surgery), IS is usually instituted. The additional staff time and equipment are justified in this high-risk group. Positive pressure therapy requires significantly more staff time and equipment and is reserved for high-risk patients who cannot perform IS techniques. The remainder of this chapter describes the use of IS and positive pressure therapy for the prevention or correction of atelectasis. The purpose of IS is to guide the patient to take a sustained maximal inspiratory effort resulting in a decrease in Ppl and maintain the patency of airways at risk for closure. Because of its simplicity, IS has been the mainstay of lung expansion therapy for many years. IS devices are designed to mimic natural sighing by encouraging patients to take slow, deep breaths. IS can be performed using devices that provide visual cues to patients when the desired inspiratory flow or volume has been achieved. IS has been shown to be an efficient and effective prophylaxis against postoperative atelectasis in high-risk patients.5 The first documented use of incentive spirometry as a therapy was in 1972, and this led to the development of a visual feedback device in 1973.7 The desired volume and number of repetitions to be performed are initially set by the RT or other qualified caregiver. The inspired volume goal is set on the basis of predicted values or observation of initial performance. The true benefit from IS is best achieved by repeated use and proper technique.8 The American Association for Respiratory Care (AARC) has developed and published a clinical practice guideline on IS; excerpts from this guideline appear in Clinical Practice Guideline 39-1. The basic maneuver of IS is a sustained maximal inspiration (SMI). An SMI is a slow, deep inhalation from the functional residual capacity (FRC) up to (ideally) the total lung capacity, followed by a 5- to 10-second breath hold. An SMI is functionally equivalent to performing an inspiratory capacity (IC) maneuver, followed by a breath hold. Figure 39-2 compares the alveolar and Ppl changes occurring during a normal spontaneous breath and an SMI during IS. Given its normal physiologic basis, IS presents few major hazards and complications; those that can occur are listed in Box 39-3. Acute respiratory alkalosis is the most common problem and occurs when the patient performs IS too rapidly. Dizziness and numbness around the mouth are the most frequently reported symptoms associated with respiratory alkalosis. This problem is easily corrected with careful instruction and monitoring of the patient. Discomfort with deep inspiratory efforts secondary to pain is usually the result of inadequate pain control in a postoperative patient. This problem can be rectified by ensuring appropriate analgesia. In addition, pain medication should be coordinated with IS activity.
Lung Expansion Therapy
 Describe the various causes of atelectasis.
Describe the various causes of atelectasis.
 Identify which patients need lung expansion therapy.
Identify which patients need lung expansion therapy.
 Define the clinical findings seen in atelectasis.
Define the clinical findings seen in atelectasis.
 Describe how lung expansion therapy works.
Describe how lung expansion therapy works.
 List the indications, hazards, and complications associated with the various modes of lung expansion therapy.
List the indications, hazards, and complications associated with the various modes of lung expansion therapy.
 Describe the primary responsibilities of the respiratory therapist in planning, implementing, and evaluating lung expansion therapy.
Describe the primary responsibilities of the respiratory therapist in planning, implementing, and evaluating lung expansion therapy.
Causes and Types of Atelectasis
Factors Associated With Causing Atelectasis
Lung Expansion Therapy
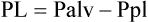
Incentive Spirometry
Physiologic Basis
Hazards and Complications
Lung Expansion Therapy

 ). Gas distal to the obstruction is absorbed by the passing blood in the pulmonary capillaries, which causes partial collapse of the nonventilated alveoli. When ventilation is compromised to a larger airway or bronchus, lobar atelectasis can develop.
). Gas distal to the obstruction is absorbed by the passing blood in the pulmonary capillaries, which causes partial collapse of the nonventilated alveoli. When ventilation is compromised to a larger airway or bronchus, lobar atelectasis can develop. predicted)
predicted)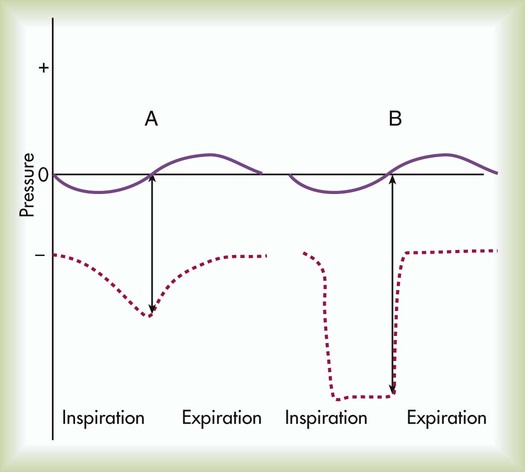
 predicted)
predicted)

