We sought to evaluate intermediate to long-term follow-up after coronary artery fistula (CAF) closure with emphasis on thrombotic complications. Transcatheter closure (TCC) or surgical closure (SC) is the current standard of treatment for significant CAF. Incidence and risk factors of coronary thrombosis after CAF closure have not been well described. Patients with CAF were identified from a departmental database and their medical records were retrospectively reviewed. CAFs were classified as proximal or distal based on origin and size as small, medium, or large. Of 16 patients, 12 underwent TCC and 4 SC. Median follow-up was 2.3 years (0.1 to 41.6). Myocardial infarction (MI) related to coronary thrombosis occurred in 3 patients; immediately, 0.9 year, and 42 years after closure. Ages at MI were 9.2, 57, and 49 years. All 3 had distal, large CAFs and underwent SC. Anticoagulation was used in 2 of 3 patients. In the remaining 13 patients, TCC was performed in 12 and SC in 1; mean age was 13.8 years (0.1 to 38.9). CAFs were proximal in 10 and distal in 3 and large in 10 and medium in 3. On follow-up, these patients were asymptomatic without MI. Anticoagulation was used in 9 of 13 after closure. In conclusion, patients with CAF are at risk for coronary artery thrombosis and MI after closure. Patients with distal type, large CAF, and older age at presentation may be at higher risk for coronary thrombosis. Close follow-up with anatomic and functional coronary evaluation is warranted in all patients after CAF closure.
Coronary artery fistulae (CAFs) are rare congenital anomalies with a reported incidence of 0.2% to 0.6%. About 75% are small and clinically silent with the possibility of spontaneous regression. Moderate to large fistulae are often symptomatic at or beyond the second decade of life. Therefore, closure in childhood is generally recommended. Transcatheter closure (TCC) or surgical closure (SC) techniques have been used based on anatomy and institutional experience/preference. Short- and intermediate-term closure rates and complications for the 2 groups are comparable. Acute and late-onset thrombosis with and without myocardial infarction (MI) has been reported after closure. However, details of patients with coronary thrombosis after CAF closure are not well documented. We reviewed the intermediate to long-term course of patients with CAF after closure at Cleveland Clinic Foundation to better understand the thrombotic complications encountered in this population.
Methods
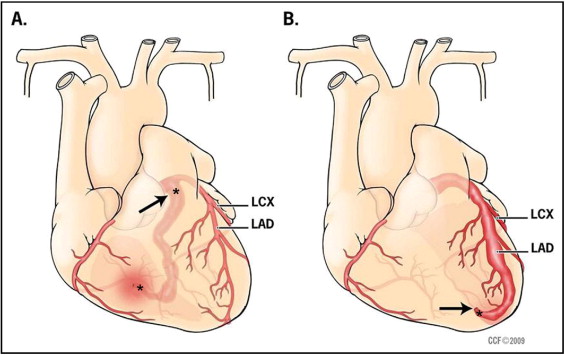
All patients who underwent CAF closure at the Cleveland Clinic Foundation from January 1997 to June 2007 were identified after institutional review board approval. We also included a patient who presented with MI during this timeframe but who had undergone CAF repair many years previously. A retrospective review of all available medical records before and after CAF closure was performed. Review included clinical evaluations, electrocardiograms, echocardiograms, catheterization images and reports, and surgical reports. All available studies detailing anatomic and functional evaluations of coronary arteries before closure and during follow-up were reviewed. Information about use of anticoagulation after closure was also collected. To identify possible risk factors for thrombotic complications after closure, CAF size was categorized as small, medium, or large as described by physicians in their report at time of intervention. CAFs were classified as proximal (arising in the first third of the central coronary artery branch) or distal. The proximal type of fistula arises near the origin of the coronary artery ( Figure 1 ). A short proximal segment of the feeding coronary artery may be dilated, but the coronary arteries distal to the fistula are of normal caliber. There are no significant coronary branches supplying the myocardium from the fistula itself. The distal type of fistula ( Figure 1 ) has its origin near the distal end of a branch coronary artery. The feeding coronary artery proximal to a distal fistula gives rise to coronary branches that supply the myocardium. Increased flow in the feeding or conduit coronary artery (fistula flow plus normal myocardial flow) results in dilation and tortuosity of the coronary artery proximal to the fistula site. Transcatheter closure techniques have been previously described. Briefly, smaller fistulae were typically closed with coils directly from an arterial approach. Large CAFs were closed preferentially by forming an arteriovenous wire loop allowing safe delivery of larger devices from a venous approach. Proximal CAFs underwent closure proximally (close to the origin) or distally at the drainage site in some and, when feasible, had proximal and distal occlusions ( Figure 1 ). For all distal CAFs extreme care was taken to place the occluding device as close to the actual fistulous opening as possible to avoid compromising flow to proximal coronary branches supplying the myocardium ( Figure 1 ). SC was performed before the development of TCC techniques or for fistulae not amenable to TCC (such as extreme tortuosity precluding distal delivery of a device or very small patient size). Ligation was performed distally at or near the draining site under cardiopulmonary bypass in all patients undergoing SC.
Results
Sixteen patients were identified who underwent CAF closure or had an MI after previous CAF repair from 1997 to 2007. Demographics, type and size of CAF, type of treatment, fistula origin, and drainage site are presented in Table 1 . Median age at closure was 1.6 years (range 0.1 to 56.3). Almost all patients had no cardiac symptoms. Only 1 patient had a history of shortness of breath, exercise intolerance, and 2 bouts of endocarditis. Three patients had associated intracardiac lesions (Tetralogy of Fallot, pulmonary atresia, mitral valve prolapse requiring mitral valve replacement) and 1 had Takayasu arteritis. In 10 patients CAFs arose from the left coronary artery and in 6 from the right coronary artery (RCA). All except 1 drained into the right side of the heart. Fistula origin was proximal in 10 and distal in 6 patients. Fistulae were large in 13 and medium sized in 3 patients. Twelve patients underwent TCC and 4 had SC. The various devices used for TCC are presented in Figure 2 . Only 1 patient in the TCC group had a small residual leak. All 4 patients who underwent SC had successful distal ligation without residual leak. One patient underwent closure in 1958 before the development of TCC techniques. At least some follow-up data were available in 15 of 16 patients ( Table 1 ) at a median of 2.3 years (0.1 to 41.6). Clinical evaluation and echocardiography were performed in all 15 patients. Most patients (12 of 15) were asymptomatic. The patient with Takayasu arteritis developed aortic insufficiency and ascending aortic aneurysm requiring surgical intervention. Echocardiogram showed complete closure in 12 patients, trivial leak in 2, and small to moderate leak in 1 patient. Exercise testing performed in only 2 patients showed normal results. One patient had a myocardial perfusion study showing a reversible perfusion defect (patient 16). Coronary artery angiogram was available in 6 patients as described in detail below.
| Patient Number | Age (years) | Origin and Drainage | Type of CAF | Size of CAF | Site of Closure | Type of Intervention/Device | Antithrombotic Prescription: Aspirin/Warfarin | Follow-Up Angiogram/Evidence of MI |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.9 | RCA to RA | proximal | large | proximal and distal | TCC/10- and 12-mm Amplatzer vascular plugs | +/0 | no residual leak or thrombosis |
| 2 | 14.9 | LCA to LV | proximal | large | proximal and distal | TCC/Amplatzer ASD 10 mm and vascular plug 8 mm | +/+ | lost to follow-up |
| 3 | 30 | LAD to RV | distal | large | distal | TCC/Amplatzer PDA occluder 10/6 | +/+ | mild residual leak with proximal persistent coronary dilatation |
| 4 | 1.3 | LCA to RA | proximal | large | proximal and distal | TCC/5-mm Flipper coil | +/0 | moderate residual leak, closure with Grifka bag, no thrombosis |
| 5 | 17.9 | RCC to RV | proximal | large | proximal | TCC/12 6-mm Tornado coils | ? | − |
| 6 | 8 | RCA to RA | proximal | large | proximal | TCC/Amplatzer PDA occluder 6/4 | +/0 | − |
| 7 | 15.9 | RCA to CS | proximal | large | proximal | TCC/Grifka bag | ? | − |
| 8 | 14 | RCA to RA | proximal | large | distal | TCC/4- × 2-mm, 3- × 2-mm Tornado coils | +/0 | − |
| 9 | 0.3 | LAD to RV | distal | medium | distal | TCC/2 3- × 2-mm Tornado coils | +/0 | no residual leak or thrombosis |
| 10 | 13 | LCx to CS | distal | large | distal | TCC/1 4- ×4-mm Gianturco coil | ? | − |
| 11 | 38.9 | aorta to RV | proximal | medium | proximal | TCC/2 Gianturco coils | +/0 | − |
| 12 | 17 | LCA to LPA | proximal | medium | proximal | TCC/8 3- × 2-mm Tornado coils | +/0 | − |
| 13 | 0.1 | LCA to LA | proximal | large | distal ligation | SC | 0/0 | − |
| 14 | 9.2 | LCA to RV | distal | large | distal ligation | SC | ? | MI at 50 years old, complete LAD occlusion |
| 15 | 52 | LCx to CS | distal | large | distal ligation | SC | +/0 | MI at 52 years old, acute postoperative period, increased enzymes, CHF, EF 40% |
| 16 | 56 | RCA to RA | distal | large | distal ligation | SC | +/+ | MI 10 months after closure, complete RCA occlusion |
Coronary artery thrombosis was observed in 2 of 15 patients (patients 14 and 16) and presumed without angiographic confirmation in patient 15 ( Table 1 ). All 3 had distal-type CAF with a massively dilated coronary artery proximal to the fistula and had SC at the drainage site. Patient 14 had a left anterior descending coronary artery to right ventricular fistula. He underwent SC in 1958 at 9 years of age. Intraoperatively he was found to have an aneurysmal left anterior descending coronary artery. The fistula was ligated distally at the drainage site in the right ventricle. He presented with MI at 50 years of age with increased enzymes and anterior ischemic electrocardiographic changes. Coronary angiogram showed complete left anterior descending coronary artery occlusion at its origin with a large calcified aneurysm of the proximal left anterior descending coronary artery measuring 2.4 cm in diameter. On echocardiogram he had mild ventricular dysfunction and was treated medically including warfarin. Patient 15 had SC of a large left circumflex coronary artery to coronary sinus fistula at 52 years of age. Echocardiogram demonstrated normal left ventricular function before CAF closure. Intraoperatively, the left main trunk and circumflex branch were noted to be massively dilated and tortuous. He had clinical evidence of MI in the early postoperative period with ischemic electrocardiographic changes, increased enzymes, and hemodynamic instability. He gradually recovered, but echocardiogram on discharge showed global hypokinesia with an ejection fraction of 40%. He was placed on longer-term warfarin therapy. At last follow-up 7 years later he remained stable with persistently impaired left ventricular function. The cause of his MI was believed to be thrombosis of the dilated proximal circumflex coronary artery. Patient 16 had a large RCA to right atrial (near the opening of the coronary sinus) fistula. She was symptomatic with breathlessness and decreased exercise tolerance before closure and had 2 bouts of endocarditis related to the fistula. She underwent SC at 56 years of age and was maintained on warfarin indefinitely due to visible stasis of blood in the dilated RCA. She presented with MI 10 months after closure. Angiogram showed complete RCA occlusion with collateral blood supply from the left coronary artery ( Figure 3 ). Adenosine stress testing 6 years after surgery showed a reversible perfusion defect and on last follow-up she was stable with medical management.




