Introduction and historical perspective
Infective endocarditis (IE) is a non-contagious, microbial infection of the valves and endocardium of the heart. The disease was first described by a French physician, Jean Francois Fernel, in his book Medicina in 1554.1 Almost 300 years later, in 1885, Sir William Osler gave a comprehensive account of endocarditis in three Gulstonian lectures.2 Osler astutely noted the host factors which predisposed to developing endocarditis, describing it “as a primary disease of the lining membrane of the heart or its valves, either attacking persons in previous good health or more often attacking the debilitated and dissipated and those with old valve lesions.”
Initially known as bacterial endocarditis, infective endocarditis (IE) is the current nomenclature used since fungi are recognized as potential causative organisms.3 Infection of arteriovenous shunts, arterio-arterial shunts (such as patent ductus arteriosus), and coarctation of the aorta, although known as infectious endarteritis, are clinically and pathologically similar to infective endocarditis. Endocarditis is classified as acute or subacute based on the rapidity of progression and presentation of the illness prior to diagnosis, factors related to both the virulence of the causative organism and host immune response. Acute endocarditis develops over days to weeks with marked toxicity and a course involving valvular destruction, extracardiac complications and, if untreated, death. In contrast, subacute endocarditis follows an indolent course, progressing slowly over weeks to months, causing less severe valvular destruction and rarely metastatic infection or extracardiac complications.4 Native valve endocarditis involves any of the four native cardiac valves, whereas prosthetic valve endocarditis involves a biologic or mechanical valve replacement or prosthetic repair material (e.g. annuloplasty ring). Prosthetic valve endocarditis is further classified into early (within 12 months of valve replacement) and late (greater than 12 months after implantation). Nosocomial endocarditis is defined as developing in a patient hospitalized for more than 48 hours before the onset of signs or symptoms consistent with endocarditis. Cardiac device infection refers to an infection of an intracardiac device such as a permanent pacemaker or implantable cardioverter-defibrillator, the infection of which may also be associated with IE.
The incidence of IE varies regionally from 2.6/100 000 population reported in France5 to 11.6/100 000 population in Philadelphia.6 This range of incidence has been attributed to differences in predisposing cardiac conditions or risk factors, such as injection drug use. The incidence of native valve endocarditis increases with age and exceeds 14.5–30/100 000 after 30 years of age. The average age of the patient with endocarditis has increased over time, likely related to the decreased prevalence of rheumatic heart disease (the major predisposing cardiac condition in Osle-rian times)7 and increased prevalence of degenerative valvular disease in the aging population. IE is more commonly diagnosed in men, with studies showing male-to-female ratios of 1.7:1.8
In earlier eras, streptococcal species were the predominant cause of native valve IE. However, changes in the delivery of healthcare, with increasing exposure to invasive procedures and devices, and the changing demographics of patients and their risk factors for endocarditis have led to major changes in the microbiologic causation of endocardits.9 In the contemporary, multinational study, the International Collaboration on Endocarditis or ICE registry (n= 1179 patients with definite native or prosthetic endocarditis), 32% cases were attributable to S. aureus, 18% viridans streptococci, 11% enterococci, 11% coagulase-negative staphylococci, 5% other streptococcal species, 2% non-HACEK gram-negative bacteria, 2% fungi, and 2% HACEK group.10 Among those with S. aureus endocarditis, healthcare-associated infection accounted for 39% of cases.10
Similarly, the microbiology of prosthetic valve endocarditis (PVE) has changed. Whereas coagulase-negative staphylococcal infection (such as S. epidermidis) was previously the most common causative species, a recent, large study of 556 cases of definite PVE found that S. aureus (23.0%) was the predominant cause of infection, with coagulase-negative staphylococci accounting for 16.9%.11
A pre-existing valvular or endocardial condition, such as mitral valve prolapse with regurgitation, degenerative aortic valve disease (including bicuspid aortic valve) and congenital heart disease, is a major host factor related to development of IE. Endothelial damage and denudation of the endothelium exposes the underlying basement membrane and fosters platelet and fibrin deposition, a process that occurs spontaneously in individuals with valvular heart disease. These deposits are called non-bacterial thrombotic endocarditis and form the nidus for vegetations in the setting of bacteremia. The ability of micro-organisms to adhere to denuded endothelium is another factor related to development of IE. The classic lesion of endocarditis, the vegetation, is thus made up of fibrin, platelets, inflammatory cells and micro-organisms, adherent to the endothelium of the heart (Plate 59.1).
The diagnosis of endocarditis is dependent on findings of bacteremia with an organism associated with endocarditis and evidence of endocardial involvement. Because these objective findings may not be sought unless the possibility of endocarditis is considered, careful attention to the patient’s history and physical examination is critical to the eventual diagnosis. The clinical presentation of IE is highly variable, and can range from chronic fatigue with low-grade fever to acute heart failure due to new, severe valvular regurgitation. While the virulence of the organism can influence acuity of presentation, the onset of infection in most cases is quickly followed by the onset of symptoms. Most patients with endocarditis develop symptoms within two weeks of bacteremia.12 In general, four processes contribute to the clinical presentation of endocarditis: (1) infection on the valve, including the local intracardiac complications; (2) septic or aseptic embolization to distant organs; (3) continuous bacteremia, often with metastatic foci of infection; and (4) circulating immune complexes and other immunopathologic factors.8
Approximately 85% patients present with fever, although this finding may not be present in immunosuppressed states and in patients who have previously been on antibiotic therapy. Non-specific symptoms such as chills (42–75%), sweats (25%), anorexia (25–55%), weight loss (25–35%), malaise (25–40%), dyspnea (20–40%), and cough (25%) are common. In addition, predisposing conditions or risk factors for the development of endocarditis, including a history of structural heart disease, injection drug use or recent invasive procedure, should be sought in the patient’s history.
Evidence of a new or changing regurgitant murmur in the presence of fever of undetermined origin should prompt further, objective evaluation for possible endocarditis. Embolic phenomena, a common extracardiac complication of endocarditis, may present with localizing symptoms.8 The patient should be carefully examined for any peripheral stigmata of endocarditis such as petechiae, splinter hemorrhages, Janeway lesions (10%), Osler nodes (10–25%) and Roth spots. Many of these findings are immune mediated. Although Janeway lesions, Osler nodes, and Roth spots are more specific abnormalities for endocarditis, they may occur in other conditions and their low frequency in cases of proven endocarditis limits their diagnostic utility for this condition.
Laboratory testing
Blood cultures
Blood cultures remain the definitive microbiologic procedure for the diagnosis of IE. Continuous and low-grade bacteremia makes it unnecessary to await fever spikes or chills to obtain blood cultures and the first two blood cultures yield an etiologic agent in 90% of cases.13 In antibioticnaïve patients, it is recommended that at least three blood culture sets from separate venepunctures should be obtained over the first 24 hours, which will increase the yield to more than 95% in cases of untreated endocarditis with continuous bacteremia (Class I, Level A). Finally, each culture media bottle should be inoculated with at least 10 mL of blood to increase the number of colony-forming units per culture (Class I, Level B). The results of blood cultures should be interpreted based on the specific micro-organisms identified as well as the recognized, constant nature of bacteremia in endocarditis.
Other laboratory data, although useful clues, are not diagnostic due to their lack of specificity. Hematologic parameters are often abnormal. A normocytic, normochromic anemia (70–90%), thrombocytopenia (5–15%) and leukocytosis (30%) dominate the peripheral blood picture. The differential cell count is usually normal, but there may be a neutrophil predominance. The erythrocyte sedimentation rate (ESR) and C-reactive protein concentrations are usually elevated.13 Rheumatoid factor assay is positive in up to half of the cases, especially if the illness is protracted.14 Urinalysis may demonstrate microscopic hematuria and mild proteinuria. Red blood cell casts and heavy proteinuria can be seen in patients with immune complex glomerulonephritis.8,13
Electrocardiography
Although the electrocardiogram lacks sufficient sensitivity and specificity for the diagnosis of endocarditis, electrocar-diographic abnormalities commonly occur in patients with endocarditis (26% in one cohort) and are associated with invasive infection and increased in-hospital mortality. The presence of atrioventricular heart block in a patient with IE is diagnostic of the presence of a ring abscess, typically of the aortic valve with invasion posteriorly toward the atrioventricular conduction system. Meine et al found that 53% of patients with invasive infection had EKG changes and about a third of the patients with EKG conduction abnormalities died during hospitalization in their cohort of 137 patients with definite endocarditis.15
Diagnostic criteria for IE
Diagnostic criteria developed by Petersdorf and Pellitier in 197713 and subsequently by Von Reyn in 198116 have been replaced by the Duke Criteria, first proposed in 1994,17 which incorporated echocardiographic findings and isolation of “typical” micro-organisms into the case definitions. Several comparative studies of the Von Reyn and Duke Criteria in various cohorts established the superior sensitivity of the Duke criteria.18–25 Recent modifications of the Duke criteria have added more specificity to the schema26 (Table 59.1). In addition to their diagnostic utility in clinical care, these criteria provide a common case definition for studies of this condition.
Table 59.1 The modified Duke Criteria and case definitions of infective endocarditis
| Modified Duke Criteria | Case definitions |
Major Criteria | Definitive Infective Endocarditis |
Blood culture positive for IE Typical microbes consistent with IE from 2 separate blood cultures: viridans streptococci, Strep bovis, HACEK group, Staph aureus, community-acquired enterococci in absence of another focus; or Micro-organisms consistent with IE from persistently positive blood cultures defined as follows: at least 2 blood cultures drawn > 12 h apart or all of three or majority of >4 separate blood cultures Single positive blood culture for Coxiella burnetti or antiphase IgG antibody titer >1:800 Evidence of endocardial involvement Echo positive for IE defined as follows: Oscillating intracardiac mass on valve or supporting structure Abscess New partial dehiscence of prosthetic valve New valvular regurgitation Minor criteria Predisposition; predisposing heart condition or injection drug use Fever, temperature >38°C Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhage, and Janeway lesions Immunologic phenomena; glomerulonephritis, Osler’ snodes, Roth’s spots, rheumatoid factor Microbiologic evidence: positive blood cultures but does not meet a major criterion as noted above, or serologic evidence of active infection with organism consistent with causing IE | Pathologic criteria 1. Micro-organisms demonstrated by culture or histologic examination of a vegetation, a vegetation that has embolized or an intracardiac abscess specimen; or 2. Pathologic lesions; vegetation or intracardiac abscess confirmed by histologic examination showing active endocarditis Clinical criteria 1. 2 major criteria; or 2. 1 major and 3 minor criteria; or 3. 5 minor criteria Possible Infective Endocarditis 1. 1 major criterion and 1 minor criterion; or 2. 3 minor criteria Rejected 1. Firm alternative diagnosis explaining evidence of infective endocarditis; or 2. Resolution of infective endocarditis syndrome with antibiotic therapy for ≤ 4 days; or 3. No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for < 4 days; or 4. Does not meet criteria for possible infective endocarditis, as above |
Adapted from Li8.
Echocardiography and other cardiac imaging modalities
Echocardiography provides not only non-invasive evidence of endocardial infection that, as described above, is among the major diagnostic criteria for endocarditis, but also important hemodynamic information regarding the presence and severity of valvular regurgitation. It is of fundamental importance in detecting vegetations, visualized as “echogenic distinct masses” from the adjacent valve with “independent motion”, from the valve itself (Fig. 59.1).
Figure 59.1 TTE parasternal long axis view demonstrating a large vegetation on the ventricular aspect of the anterior mitral valve leaflet extending into the left ventricular outflow tract.
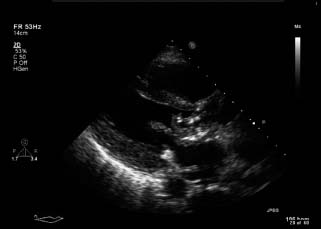
The diagnostic utility of transthoracic echocardiography (TTE) for suspected IE is highest in patients with intermediate to high likelihood of this disease27 (e.g. a patient with a new or changed heart murmur and bacteremia). Hence, TTE should be performed in all patients with suspected IE (Class I, Level A). However, the diagnostic sensitivity of TTE for the visualization of an intracardiac vegetation or abscess is limited, ranging from 40% to 80%, and thus the diagnosis of endocarditis cannot be “ruled out” on the basis of a negative study.
Transesophageal echocardiography (TEE) has greater spatial resolution than TTE and thus is more sensitive than TTE for the detection of intracardiac vegetations As a result, TEE should be performed in patients with a high likelihood of IE and a negative TTE28,29 (Class I, Level A) (Plate 59.2). Although TTE and TEE have been found to have concordant results in approximately half of patients with suspected endocarditis, TEE results in additional diagnostic information in a high percentage, particularly those with prosthetic valves.30 Specific subsets of patients in which TEE should be performed, even as the primary imaging modality (without TTE) include those with prosthetic heart valves and suspected endocarditis (Class I, Level B) and those with persistent staphylococcal bactere-mia without known source (Class IIa, Level B) or nosoco-mial staphylococcal bacteremia (Class IIb, Level C). For instance, TEE has been shown to be a cost-effective method for determining the duration of antibiotic therapy (two weeks vs four weeks) compared to empiric duration in patients with intravascular catheter associated S. aureus bacteremia.31 TEE has a high sensitivity and specificity (87% and 95%, respectively) and therefore should be performed in patients with endocarditis when perivalvular abscess is suspected (Class I, Level A).32
Cardiac magnetic resonance imaging with contrast appears promising for the detection of perivalvular abscesses, thrombus associated with vegetations, valvular complications and aortocameral fistulas, although temporal resolution may limit its use for detection of vegetation (Level C). Cardiac computed tomography has also been used to detect aortic root abscess. However, clinical experience with these techniques in IE patients is limited and their operating characteristics (sensitivity and specificity) in comparison to echocardiography are not well defined.33–35
Routine coronary angiography is recommended in patients over age 55 prior to surgery for IE or in those at high risk for coronary artery disease.
The management of IE has evolved over the last few decades with improvements in diagnostic capabilities and therapy.17,32 Rapid diagnosis, early risk stratification, institution of appropriate bactericidal therapy, and prompt recognition and treatment of complications are key elements in a good outcome. Central to the care of these complex patients is the involvement and close collaboration of multiple interdisciplinary teams including the general medicine team, infectious diseases specialists, cardiologists, and cardiothoracic surgeons.
Antibiotic therapy
Antibiotic therapy has improved survival in IE by 70–80%, and has been shown to reduce the incidence of complications of endocarditis. Although the choice of antimicrobial therapy is mainly guided by the infecting organism and its antibiotic susceptibilities, there are three basic tenets of treatment that are aimed at eradicating the infecting organism from vegetations.
First, a prolonged course of antibiotic treatment (4–6ix weeks) is necessary to eradicate infection because bacterial concentration within vegetations is as high as 109 to 1011
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


