Infective Endocarditis
Jennifer S. Li
G. Ralph Corey
Vance G. Fowler Jr.
Infectious endocarditis (IE) denotes infection of the endocardial surface of the heart and implies the physical presence of microorganisms in the lesion. Although the heart valves are most commonly affected, the disease may also occur within septal defects or on the mural endocardium. Infections of arteriovenous shunts and of arterioarterial shunts (patent ductus arteriosus) as well as infection related to coarctation of the aorta can also be included in this definition because of their similar clinical manifestations. Unfortunately, variability in the clinical presentation continues to make the diagnosis of IE challenging. Diagnostic criteria have been recently developed that utilize clinical findings, pertinent laboratory data, and echocardiography to diagnose IE with a high degree of sensitivity and specificity. Transesophageal echocardiography (TEE) has become a major advance in evaluating vegetations particularly in patients with prosthetic valve disease and in those with complications such as annular abscesses and intracardiac fistulae.
Yet, despite improvements in diagnosis and treatment, IE continues to be associated with high morbidity and mortality. Reasons for this are several. The patient with IE is often increasingly complex. For example, patients are often older with nosocomial bloodstream infections, infected prosthetic valves, or even congenital heart disease. In addition, Staphylococcus aureus has become a major pathogen in nosocomial IE. Targeted antibiotic treatment is the ideal approach to the pharmacologic management of IE. Prevention remains the standard of care. Successful management depends on the close cooperation of cardiologists, cardiothoracic surgeons, and infectious disease specialists.
Glossary
Endarteritis
Inflammation of the intima of an artery.
Janeway lesion
Erythematous, nontender lesions on fingers, palm, or sole.
Mycotic aneurysms
Aneurysmal dilatation of a vessel caused by invasion of the vascular wall from infective endocarditis.
Osler nodes
Small, tender, purple erythematous subcutaneous nodules visibly found on the pulp of digits.
Roth spots
Retinal hemorrhages with a clear center.
Splinter hemorrhage
An embolic subungual hemorrhage located proximally in the nail bed.
Vegetations
Growth consisting of fused platelets, fibrin, and other bacteria adherent to a heart valve or other vascular structure.
Historical Perspective
The first description of infection of the heart valves in humans appeared over 300 years ago (1). Riveriere described autopsy findings associated with IE in 1723 and Morgagni provided classical descriptions in 1761. In 1806, Jean Nicolause Corvisart first used the term vegetations to describe the characteristic macroscopic excrescences seen in IE (2). Laennec, Bouillaud, Charcot, and Vichow described many clinical and pathological features of IE over the next 75 years. However, it was not until 1937 that Gross concluded that heart valves were avascular structures involved in an inflammatory response (3).
Experiments by Rosenbach, Wyssokowitsch, and Weichselbaum subsequently demonstrated that most patients with IE had preexisting damage to their heart valves and that the initiating event was the entry of microbes into the bloodstream from a site of local infection or a mucosal surface (2).
Experiments by Rosenbach, Wyssokowitsch, and Weichselbaum subsequently demonstrated that most patients with IE had preexisting damage to their heart valves and that the initiating event was the entry of microbes into the bloodstream from a site of local infection or a mucosal surface (2).
In his series of famous Gulstonian lectures in 1885, William Osler became the first person to systematically describe the clinical features of IE by identifying four cardinal manifestations. These manifestations remain the hallmarks of clinical diagnosis in the present era: persistent bacteremia, active valvulitis, large-vessel emboli, and immunologic vascular phenomena (4). He coined the term mycotic aneurysm to describe the focal endarteritis that may complicate IE and described emboli to multiple organs such as the brain, spleen, kidney, and retina. He also described the Osler nodes, “small swollen areas, some the size of a pea, others a centimeter and a half in diameter, raised, red with a whitish spot in the center … the commonest situation is near the tip of the finger” (5).
A famous early case of IE was reported by Soma Weiss, who described a medical student who had chronic rheumatic heart disease and then developed S. viridans IE. In his diary, the student noted clubbing of his finger beds and peripheral embolic phenomenon. On discovering these cutaneous manifestations of his disease, he turned to his sister-in-law and prophetically proclaimed, “I shall be dead in 6 months” (6).
Although invariably fatal until the discovery of penicillin and other antimicrobial agents, steady advances in medical and surgical therapy have had a large impact on this disease. New methods in echocardiography and imaging as well as molecular tools have improved the ability to diagnose IE. However, the heterogeneous population of patients who develop IE and the growing development of resistant organisms continue to make IE a challenging disease for clinicians.
Epidemiology
The incidence of IE is difficult to determine because the criteria for diagnosis and the methods of reporting vary with different series (7,8). An analysis based on strict case definitions often reveals that only a small proportion (∼20%) of clinically diagnosed cases are categorized as definite. Nevertheless, IE accounted for approximately 1 case per 1,000 hospital admissions, with a range of 0.16 to 5.40 cases per 1,000 admissions, in a review of 10 large surveys (7,9). This incidence has not changed appreciably over the past 30 years (10). Estimates from the American Heart Association (AHA) place the annual incidence of IE in the United States at 10,000 to 20,000 new cases.
Men are more commonly affected than women (mean male: female ratio 1.7:1 in 18 large series) (11). However, in patients under the age of 35 years, more cases occur in women. The disease remains uncommon in children and infants, in whom it is associated primarily with nosocomial bacteremia in the setting of underlying structural congenital heart disease (12,13).
There is general agreement that new trends in the epidemiology of IE have occurred during the past 30 years. These changes are mainly due to the types of susceptible hosts rather than to shifts in the virulence of the infecting microorganisms. Patients with IE are typically older. In 1926, the median age was less than 30 years; this had increased to 39 years by 1943, and currently over 50% of the patients are older than 50 years (14,15,16). A number of factors may relate to this shift in age distribution. First, there has been a change in the nature of the underlying heart disease owing to a decline in the incidence of acute rheumatic fever and rheumatic heart disease and a simultaneous rise in the frequency of degenerative heart disease. Second, the age of the population has been steadily increasing, and people with rheumatic or congenital heart disease are surviving longer.
In addition, such patients increasingly undergo prosthetic valve surgery, an important risk factor for IE. More than 150,000 heart valves are implanted annually worldwide (17). Prosthetic valve IE (PVE) develops in 1% to 4% of prosthetic valve recipients in the first year following valve replacement and in approximately 1% of recipients annually thereafter (18,19). The risk of IE in patients with mechanical or bioprosthetic valves is similar. In a multicenter follow-up study of over 1,000 patients who were randomized to receive mechanical or bioprosthetic valves, the overall rate of PVE was similar in both groups (0.8 cases per year of follow-up) (18). Mechanical prosthetic valves may be more susceptible to IE initially, whereas after 1 year, bioprosthetic valves are more likely to develop IE (20,21).
A new form of the disease—nosocomial IE—secondary to new therapeutic modalities (intravenous catheters, hyperalimentation lines, pacemakers, dialysis shunts, etc.) has emerged (22). Nosocomial IE is usually a complication of bacteremia induced by an invasive procedure or a vascular device. Of 125 cases of IE reviewed in Seattle, 35 were nosocomial in origin (28%) (23). Although nosocomial IE accounted for only 14.3% of cases in another recent study, 64% of patients were over 60 years of age, and mortality was high (24). The emerging importance of nosocomial IE in industrialized nations has influenced the microbiology of IE, with an increasing prevalence of S. aureus and decreasing prevalence of viridans streptococci among U.S. tertiary care centers (22). S. aureus is now a leading cause of bacteremia and IE. Over the past several years, the frequency of S. aureus bacteremia (SAB) has increased dramatically. This increasing frequency, coupled with increasing rates of antibiotic resistance, has renewed interest in this serious, common infection. S. aureus is a unique pathogen because of its virulent properties, protean manifestations, and ability to cause IE on architecturally normal cardiac valves (25).
The rise in the incidence of injection drug use has also had a major impact on the epidemiology of IE. The incidence of IE in intravenous drug users (IDU) may be 30 times higher than the general population and 4 times higher than the risk of IE in adults with rheumatic heart disease (26). In some areas of the United States, drug addiction is the most common predisposing cause for IE in patients less than 40 years old (27). S. aureus is the predominant organism. Tricuspid valve involvement is noted in 78%, mitral in 24%, and aortic in 8% of drug abusers with IE (28). More than one valve is involved in approximately 20% of cases and some of these infections are polymicrobial (29,30).
Hemodynamic and Anatomic Considerations
IE characteristically occurs on the atrial surface of the atrioventricular and the ventricular surface of the semilunar valve when associated with valvular insufficiency. Rodbard (31) showed that this localization is related to a decrease in lateral pressure (presumably with decreased perfusion of the intima) immediately “downstream” from the regurgitant flow. Lesions with high degrees of turbulence (small ventricular septal defect with a jet lesion, high-velocity jets across stenotic or regurgitant valves) readily create conditions that lead to bacterial colonization, whereas defects with a large surface area (large ventricular septal defect), low flow (ostium secundum atrial septal defect), or attenuation of turbulence (chronic congestive heart failure [CHF] with atrial fibrillation) are rarely implicated in IE. Cures of IE achieved with ligation alone of an arteriovenous fistula
or patent ductus arteriosus also highlight the importance of hemodynamic factors.
or patent ductus arteriosus also highlight the importance of hemodynamic factors.
The degree of mechanical stress exerted on the valve also affects the location of the IE (32). In 1,024 autopsy cases of IE reviewed through 1952, the incidence of valvular lesions was as follows: mitral, 86%; aortic, 55%; tricuspid, 19.6%; and pulmonic, 1.1%. This correlates with the pressure resting on the closed valve: 116, 72, 24, and 5 mm Hg, respectively.
Previous studies have shown that approximately three fourths of all patients with IE have a preexisting structural cardiac abnormality at the time that IE begins (33). However, the increasing incidence of S. aureus in nosocomial and IDU-associated IE has altered this pattern.
Aortic valve disease has been a predisposing cause for IE in 12% to 30% of cases (34). The peak gradient across the aortic valve is linked to the risk of IE; the higher the gradient, the higher the risk of developing IE (35). Patients with aortic regurgitation have an approximately 50% lower incidence of IE than those with aortic stenosis (36). Mitral valve disease has become relatively more important as a predisposing cause for IE. Preexisting mitral valve prolapse was the underlying cardiac lesion in 22% to 29% of IE in two cases series (37,38). The risk of IE is clearly higher in patients with mitral valve prolapse. In a careful retrospective epidemiologic matched case-control analysis, the calculated odds ratio (OR) 8.2 (95% confidence interval [CI], 2.4–28.4) indicates a substantially higher risk for the development of IE in these patients than in controls (39). Historically, rheumatic heart disease was the underlying lesion in 37% to 76% of the infections, and the mitral valve is involved in more than 85% of these cases (40). The tricuspid valve is rarely involved (0% to 6% of the cases), and the pulmonary valve even less often (<1%).
Congenital heart disease is now the underlying lesion in 10% to 20% of cases of IE (41). The most common congenital heart lesions predisposing to IE include bicuspid aortic valve, patent ductus arteriosus, ventricular septal defect, coarctation of the aorta, and tetralogy of Fallot. Unlike most other congenital defects, secundum atrial septal defects are not associated with an increased risk (42).
IE in patients with hypertrophic cardiomyopathy is virtually confined to those patients with outflow obstruction. In the subset of patients with hypertrophic cardiomyopathy who have outflow obstruction, the incidence of IE is 3.8 per 1,000 person-years with a probability of IE of 4.3% at 10 years. In patients with both outflow obstruction and left atrial dilatation (≥50 mm), the incidence of IE increases to 9.2 per 1,000 person-years (43).
The “degenerative” cardiac lesions (calcified mitral annulus, calcific nodular lesions secondary to arteriosclerotic cardiovascular disease) assume the greatest importance in patients without any demonstrable underlying valvular disease. The actual contribution made by these lesions is unknown, but they occur with an increased incidence in the elderly. In one series, degenerative lesions were present in 50% of patients over 60 years old with native valve IE (44).
Pathophysiology
The endothelial lining of the heart and its valves is normally resistant to infection with bacteria and fungi. In vitro observations and studies in experimental animals have demonstrated that the development of IE requires the simultaneous occurrence of several independent events, each of which may be influenced by a host of separate factors (45,46,47,48). The valve surface must first be altered to produce a suitable site for bacterial attachment and colonization. Surface changes may be produced by various local and systemic stresses, including blood turbulence. These alterations result in the deposition of platelets and fibrin and in the formation of so-called sterile vegetation—the lesions of nonbacterial thrombotic endocarditis (NBTE).
Bacteria must then reach this site and adhere to the involved tissue to produce colonization. Transient bacteremia occurs whenever a mucosal surface heavily colonized with bacteria is traumatized, such as with certain dental, gastrointestinal, urologic, and gynecologic procedures. Certain strains of bacteria appear to have a selective advantage in adhering to platelets and/or fibrin and thus produce the disease with a lower inoculum. The ability of these organisms to adhere to NBTE lesions is a crucial early step in the development of IE. Gould et al. (49) showed that organisms frequently associated with IE (enterococci, viridans streptococci, S. aureus, S. epidermidis, Pseudomonas aeruginosa) adhered more avidly to normal canine aortic leaflets in vitro than did organisms uncommon in IE (Klebsiella pneumoniae, Escherichia coli) (49). In addition, S. aureus and the viridans streptococci produced IE more readily than did E. coli in the rabbit model of IE (50). This observation correlates with the relative frequency with which these organisms produce the disease in humans. Microbial adherence is mediated by several factors, including the amount of dextran in the cell wall of the microorganism, the ability of the organism to bind fibronectin, the presence of surface adhesions such as Fim A, and the presence of other compounds that may mediate bacterial adherence, including fibrinogen, laminin, and type 4 collagen (51,52,53,54).
After colonization, the surface is rapidly covered with a protective sheath of platelets and fibrin to produce an environment conducive to further bacterial multiplication and vegetation growth. Microbial growth results in the secondary accumulation of more platelets and fibrin until a macroscopic excrescence or vegetation is present (Fig. 26.1). The culmination of this process is mature vegetation consisting of an amorphous collection of fibrin, platelets, leukocytes, red blood cell debris, and dense clusters of bacteria. The surface of most vegetations consists of fibrin and scant numbers of leukocytes. Clumps of bacteria, histiocytes, and monocytes are usually found deep within the vegetation. Giant cells containing phagocytic bacteria may be found in some vegetations. Extremely high concentrations of bacteria (e.g., 109–1011 bacteria per gram of tissue) may accumulate deep within vegetations. Some of these bacteria exist in a state of reduced metabolic activity. Following
therapy and during the process of healing, capillaries and fibroblasts appear within vegetations, but without treatment, vegetations are avascular structures (55).
therapy and during the process of healing, capillaries and fibroblasts appear within vegetations, but without treatment, vegetations are avascular structures (55).
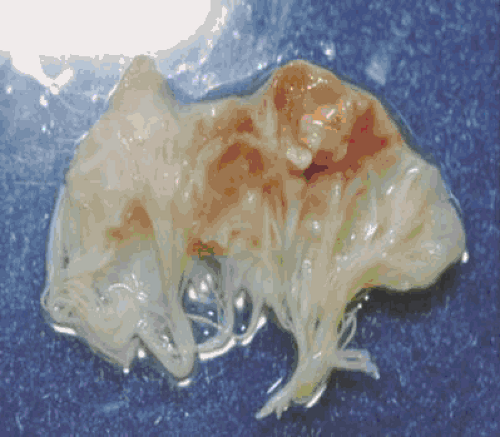 FIGURE 26.1. Pathologic specimen of a mitral valve and vegetation infected with S. aureus. Courtesy of Vance G. Fowler, Jr. MD. |
Vegetations often prevent proper valvular leaflet or cusp coaptation, resulting in worsening valvular incompetence and CHF. Vegetation growth may result in leaflet perforation that can manifest as acute CHF (56). Patients with mitral or tricuspid valve vegetations may develop chordal rupture when infection progresses beyond the valve orifice. Extension of infection may also occur into surrounding structures such as the valve ring, the adjacent myocardium, the cardiac conduction system, or the mitral–aortic intravalvular fibrosa (57). Rarely, cavitation of periaortic abscesses may occur into the adjacent aortic wall, resulting in the formation of a diverticulum or aneurysm. Even more rarely, such aneurysms may perforate into surrounding structures resulting in aortic-atrial or aortic–pericardial fistulae (58).
IE causes the stimulation of both humoral and cellular immunity as manifested by hypergammaglobulinemia, splenomegaly, and the presence of macrophages in the peripheral blood. Rheumatoid factor (anti-immunoglobulin (Ig) G IgM antibody) develops in about 50% of patients with IE of longer than 6 weeks duration (59). Antinuclear antibodies also occur in IE and may contribute to the musculoskeletal manifestations, low-grade fever, or pleuritic pain (60). Opsonic (IgG), agglutinating (IgG, IgM), and complement-fixing (IgG, IgM) antibodies and cryoglobulins (IgG, IgM, IgA, C3, fibrinogen), various antibodies to bacterial heat shock proteins, and macroglobulins all have been described in IE (61,63). Circulating immune complexes have been found in high titers in virtually all patients with IE and may cause a diffuse glomerulonephritis (64). Some of the peripheral manifestations of IE, such as Osler nodes, may also result from a deposition of circulating immune complexes. Pathologically, these lesions resemble an acute Arthus reaction. However, the finding of positive culture aspirates in Osler nodes suggests that they may in fact be due to septic emboli rather than immune complex deposition (65).
Clinical Manifestations and Complications
The clinical presentation of IE ranges from subtle, chronic fatigue with low-grade fevers, weight loss, and malaise, to an abrupt onset of fulminant acute pulmonary edema brought on by massive acute aortic regurgitation. Although the virulence of the infecting organism can influence the acuity of the presentation, the interval from onset of infection to onset of symptoms is usually short. Most patients with IE develop symptoms within 2 weeks of the inciting bacteremia (66). Symptoms in staphylococcal IE may even begin within a few days of the onset of infection.
The symptoms and signs are protean, and essentially any organ system may be involved. Four processes contribute to the clinical picture: (a) the infectious process on the valve, including the local intracardiac complications; (b) septic or aseptic embolization to virtually any organ; (c) constant bacteremia, often with metastatic foci of infection; and (d) circulating immune complexes and other immunopathologic factors (67,68). As a result, the clinical presentation of patients with IE is highly variable and the differential diagnosis often broad. Because of the protean manifestations, the diagnosis of IE may be delayed or occasionally not clinically suspected and identified only on post-mortem examination (69,70).
In more recent years, because of a higher incidence of nosocomial IE and more rapid diagnostic modalities, the patient with low-grade fever, malaise, and peripheral stigmata from long-standing IE is not as common a presentation as it was previously. Fever is usually present in the current era, but may be absent (5% of the cases), especially in the setting of CHF or other comorbid condition, immunosuppressive therapy, advanced age, or previous antibiotic therapy (71,72). Nonspecific symptoms such as anorexia, weight loss, malaise, fatigue, chills, weakness, nausea, vomiting, and night sweats are not unusual. These nonspecific symptoms may result in an incorrect diagnosis of malignancy, collagen vascular disease, tuberculosis, or other chronic disease.
Audible heart murmurs occur in over 85% of the cases, but may be absent with right-sided or a mural infection. The classic “changing murmur” and the development of a new regurgitant murmur (usually aortic insufficiency) are uncommon and occur in 5% to 10% and in 3% to 5% of the cases, respectively. When present, these are diagnostically useful signs and usually complicate acute staphylococcal disease. Over 90% of patients who demonstrate a new regurgitant murmur develop CHF. The incidence of CHF appears to be increasing (approximately 25% in 1966 and 67% in 1972) and is now the leading cause of death in IE (23). Unless valve replacement or repair is undertaken, most patients with this complication die even if effective antimicrobial therapy is administered. Although valvular regurgitation is the most important hemodynamic complication of IE, hemodynamically significant valvular obstruction requiring surgery may occur rarely, even without a prior history of valvular stenosis (73).
The classic peripheral manifestations were previously found in up to one half of the cases with IE of prolonged duration; however, the prevalence has significantly decreased in recent years. Clubbing may be present if the disease is of long duration, and may recede with therapy. The complete syndrome of hypertrophic osteoarthropathy is rare. Splinter hemorrhages are linear red to brown streaks in the fingernails or toenails. They are a nonspecific finding and are often seen in the elderly or in people experiencing occupation-related trauma. Petechiae are found after a prolonged course, and usually appear in crops on the conjunctivae, buccal mucosa, palate, and extremities. These lesions are initially red and nonblanching, but become brown and barely visible in 2 to 3 days. Petechiae may result from either local vasculitis or emboli. Osler nodes are small, painful, nodular lesions usually found in the pads of fingers or toes and occasionally in the thenar eminence. They are 2 to 15 mm in size and are frequently multiple and evanescent, disappearing in hours to days. Osler nodes are rare in acute cases of IE. They are not specific for IE because they may be seen in systemic lupus erythematosus, marantic endocarditis, hemolytic anemia, and gonococcal infections and in extremities with cannulated radial arteries. Janeway lesions are hemorrhagic, macular, painless plaques with a predilection for the palms or soles. They persist for several days and are thought to be embolic in origin. They occur with greater frequency in staphylococcal IE. Roth spots are oval, pale, retinal lesions surrounded by hemorrhage and are usually located near the optic disk. They occur in less than 5% of the cases of IE and may also be found in anemia, leukemia, and connective tissue disorders such as systemic lupus erythematosus. Splenomegaly is more common in patients with IE of prolonged duration. Splenic septic emboli are common during IE, but localized signs and symptoms may be absent in approximately 90% of patients with this complication (74).
Abscess in or adjacent to the valve annulus is often heralded by the appearance of first- or second-degree heart block and/or fever that persists despite appropriate therapy. Annular abscesses are more common in patients with aortic valvular infection than in those with mitral valve involvement. Pericarditis is rare but, when present, is usually accompanied by myocardial abscess formation as a complication of staphylococcal infection. Myocarditis may occur as a result of coronary
vasculitis, embolic coronary occlusion, or the effects of microbial toxins or immune complex deposition.
vasculitis, embolic coronary occlusion, or the effects of microbial toxins or immune complex deposition.
Musculoskeletal manifestations are common in IE; 44% of the patients in one series demonstrating musculoskeletal symptoms (75). These symptoms usually occurred early in the disease and were the only initial complaint in 15% of the cases. They included proximal oligo- or monoarticular arthralgias (38%), lower extremity mono- or oligoarticular arthritis (31%), low back pain (23%), and diffuse myalgias (19%). The back pain may be severe, limiting movement, and is the initial complaint in 5% to 10% of cases.
Major embolic episodes, as a group, are second only to CHF as a complication of IE and occur in at least one third of cases. Splenic artery emboli with infarction may result in left upper quadrant abdominal pain with radiation to the left shoulder, a splenic or pleural rub, or a left pleural effusion. Renal infarctions may be associated with microscopic or gross hematuria, but renal failure, hypertension, and edema are uncommon. Retinal artery emboli are rare (occurring in <2% of cases) and may be manifested by a sudden complete loss of vision. Pulmonary emboli can be a complication of right-sided IE. Coronary artery emboli usually arise from the aortic valve and may cause myocarditis with arrhythmias or myocardial infarction. Major vessel emboli (affecting the femoral, brachial, popliteal, or radial artery) are more frequent in fungal endocarditis.
Neurologic manifestations occur in 20% to 40% of the cases and may dominate the clinical picture, especially in staphylococcal IE. Stroke is the most common neurologic complication of IE, occurring in 9.6% of patients. Patients with mitral valve IE have a greater risk of stroke than patients with aortic valve IE (OR 2.0, 95% CI 1.1–3.9) (76). Of those patients with neurologic complications, up to 50% present with neurologic signs and symptoms as the heralding features of their illness (77,78). The development of clinical neurologic deterioration during IE is associated with a two- to fourfold increase in mortality. Mycotic aneurysms of the cerebral circulation occur in 2% to 10% of the cases. They are usually single, small, and peripheral, and may lead to devastating subarachnoid hemorrhage. Other features include seizures, severe headache, visual changes (particularly homonymous hemianopsias), choreoathetoid movements, mononeuropathy, and cranial nerve palsies. A toxic encephalopathy with symptoms ranging from a mild change in personality to frank psychosis may occur, especially in elderly patients.
Patients with IE may have symptoms of uremia. In the preantibiotic era, renal failure developed in 25% to 35% of the patients, but presently fewer than 10% are affected. When uremia does develop, diffuse glomerulonephritis with hypocomplementemia is usually found, but focal glomerulonephritis has also been implicated. Renal failure is more common with long-standing disease, but is usually reversible with appropriate antimicrobial treatment alone.
Laboratory Testing
Blood Cultures
The blood culture is the single most important laboratory test performed in a diagnostic workup for IE. The bacteremia is usually continuous and low-grade (80% of the cases have less than 100 CFU/mL of blood) (79). In approximately two thirds of the cases, all blood specimens drawn yield positive results on culture (23). When bacteremia is present, the first two blood cultures yield the etiologic agent more than 90% of the time. The sensitivity of blood cultures for the detection of streptococci is particularly susceptible to prior antibiotic therapy and is also affected by the media employed (80). Continuous monitoring blood culture systems (e.g., BACTEC, BacT/ALERT) are significantly more sensitive than conventional methods (81). In addition, blood culture media containing neutralizing resin particles have improved the detection of staphylococci from patients receiving antimicrobial therapy at the time of culture (82).
On the basis of these studies, the following procedures for culturing blood are recommended. At least three blood culture sets (no more than two bottles per venipuncture) should be obtained in the first 24 hours. More specimens may be necessary if the patient has received antibiotics in the preceding 2 weeks. Blood cultures drawn within 4 hours may yield equal results to those drawn 12 to 24 hours apart. In general, culture of arterial blood offers no advantage over use of venous blood. The constancy of bacteremia in patients with IE makes it unnecessary to await fever spikes or chills to obtain blood cultures. At least 10 mL of blood in adults or 0.5 to 5.0 mL from infants and children (when feasible) should be injected into both trypticase soy (and brain–heart infusion) and thioglycolate broth (83). Supplementation with 15% sucrose (in an attempt to isolate cell wall–deficient forms) or the use of pre-reduced anaerobic media is unrewarding. Inspection for macroscopic growth should be performed daily and routine subcultures done on days 1 and 3. The cultures should be held for at least 3 weeks. When gram-positive cocci grow on the initial isolation but fail to grow on subculture, nutritionally variant (thiol-dependent) streptococci should be suspected (84). In this event, subculture inoculation should be onto media supplemented with either 0.05 to 0.1% L-cysteine or 0.001% pyridoxal phosphate.
The interpretation of positive blood cultures requires consideration of their likelihood of causing IE. The following organisms are considered to be likely causes of IE when isolated from two or more blood cultures: S. aureus, viridans streptococci, enterococci (if acquired in the community and not nosocomially), S. sanguis, and group G streptococci. False-positive results are likely to be present when organisms such as Propionibacterium spp, Corynebacterium spp, Bacillus spp, and coagulase-negative staphylococci are recovered from a single blood culture or a minority of blood culture results. However, because these organisms are also capable of causing IE, it is important to determine if there is persistent bacteremia present as opposed to contamination with skin flora. Persistent bacteremia is likely if (a) positive cultures form organisms likely to cause IE are obtained from two samples collected more than 12 hours apart or (b) if for all other organisms, all of three or a majority of four or more separate blood cultures are positive and if the first and last samples are collected at least 1 hour apart (85).
Other Blood Laboratory Tests
The utility of other blood laboratory tests in the diagnosis of IE is limited. Hematologic parameters are often abnormal in IE, but none is diagnostic. Anemia is nearly always present (70%–90% of cases), especially in subacute cases, and has the characteristics of the anemia of chronic disease, with normochromic, normocytic indices. Thrombocytopenia occurs in 5% to 15% of the cases and leukocytosis is present in 20% to 30% of cases. The differential count is usually normal, but there may be a slight shift to the left. The erythrocyte sedimentation rate (ESR) is nearly always elevated (90% to 100% of cases), with a mean value of 57 mm/hour found in one large series (23). Hypergammaglobulinemia is detected in 20% to 30% of cases and may be accompanied by a plasmacytosis in the bone marrow aspirate. A positive result on assay for rheumatoid factor is found in 40% to 50% of cases, especially when the duration of the illness is longer than 6 weeks (58). Hypocomplementemia
(seen in 5%–15% of cases) parallels the incidence of abnormal renal function test results (elevated creatinine level in 5%–15%). Urinalysis is frequently abnormal; proteinuria occurs in 50% to 65% of cases, and microscopic hematuria in 30% to 60%. Red cell casts may be seen in as many as 12% of cases; gross hematuria, pyuria, white cell casts, and bacteriuria may also be found (23).
(seen in 5%–15% of cases) parallels the incidence of abnormal renal function test results (elevated creatinine level in 5%–15%). Urinalysis is frequently abnormal; proteinuria occurs in 50% to 65% of cases, and microscopic hematuria in 30% to 60%. Red cell casts may be seen in as many as 12% of cases; gross hematuria, pyuria, white cell casts, and bacteriuria may also be found (23).
Circulating immune complexes and mixed-type cryoglobulins are detectable in most patients with IE, but also constitute a nonspecific finding. The C-reactive protein concentration is virtually always elevated in IE and is a nonspecific finding. Recently, procalcitonin has been shown to be a better diagnostic marker than C-reactive protein in patients with suspected IE (86).
Culture-Negative Infective Endocarditis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


