CHAPTER 12 Infectious Lung Diseases
BACTERIAL LUNG INFECTIONS
Community-Acquired Pneumonia
The term pneumonia refers to infection of the lower respiratory tract, involving the respiratory bronchioles to the distal alveoli. These illnesses have been subclassified on the basis of the mode and timing of presentation and the existing comorbidities of the affected patient. These distinctions are often useful in considering empiric antibiotic therapy. The terms typical pneumonia and atypical pneumonia, which refer to the causative organism, are outdated but still occasionally useful in considering this subject. Pneumonias due to so-called atypical organisms (including Mycoplasma pneumoniae, Legionella species, and Chlamydia pneumoniae) can represent up to 40% of cases but are rarely distinguishable from typical bacterial pneumonias at clinical presentation.1 Thus, the initial therapy chosen should consider both typical and atypical pathogens.
Community-acquired pneumonia (CAP) describes an acute bacterial or viral respiratory infection contracted outside the confines of the hospital or long-term care facility setting.2,3 It remains a common illness, with an estimated incidence between 5 and 6 million cases, resulting in more than 1 million hospitalizations annually.4,5 Because CAP is not a reportable illness, these figures probably underestimate the actual magnitude of the problem. The aggregate cost for treatment of these patients approaches $9 billion a year, almost all due to the costs associated with inpatient management.5 The length of hospitalization has been shown to be the key factor in determining the cost of treatment.6 Pneumonia remains the sixth leading cause of death in the United States. The mortality rate associated with the development of pneumonia ranges from 1% to 5% for ambulatory patients without comorbidities to close to 40% for those requiring intensive care unit (ICU) admission.7
The most common pathogens responsible for CAP are listed in Table 12-1. Streptococcus pneumoniae has remained the most commonly identified organism, followed by Haemophilus influenzae, Staphylococcus aureus, enteric gram-negative bacilli, Legionella species, Mycoplasma pneumoniae, Chlamydia pneumoniae, and respiratory viruses.3,4,8 In up to 50% of cases, a causative organism is not identified. The likelihood of finding a particular pathogen often depends on the population studied (see Table 12-1) and the diagnostic tests used. For example, sputum culture favors pneumococcus as the most frequently identified organism, whereas M. pneumoniae is seen more commonly with serologic testing. A “mixed” infection involving atypical and typical organisms can approach 40%,9 although it is uncertain whether the atypical organisms, when present, represent an actual coinfection, a preceding infection, or simple colonization. However, empiric antibiotic regimens that cover both typical and atypical organisms have been associated with improved survival and shorter hospitalizations in several large retrospective studies.10–12
Certain risk factors may predispose patients to infection with particular organisms. For example, pneumonia due to drug-resistant pneumococcus has been associated with advanced age (>65 years), alcoholism, immune suppressive illness or therapy, recent β-lactam therapy, and other significant comorbidities. Enteric gram-negative infections are seen more frequently in nursing home patients, in those with other significant medical conditions including cardiopulmonary disease, and with recent antibiotic therapy. Pseudomonal infections are more likely to arise in those with structural lung disease, with malnutrition, or in the presence of immune suppressive or broad-spectrum antibiotic therapy.4 H. influenzae infections occur more frequently in smokers than in nonsmokers.
Clinical Presentation
Patients with CAP typically present with cough (90%), dyspnea (66%), sputum production (66%), and pleuritic chest pain (50%). Nonrespiratory symptoms such as malaise, headache, nausea, myalgias, arthralgias, abdominal pain, and mental confusion are seen in 10% to 30% of patients. Elderly patients often have fewer or less severe symptoms than younger patients do. Presenting signs on physical examination include fever (80%), tachypnea, tachycardia, and a generalized toxic state. Crackles on auscultation are common (80%), with indications of consolidation in up to 30% of patients.13
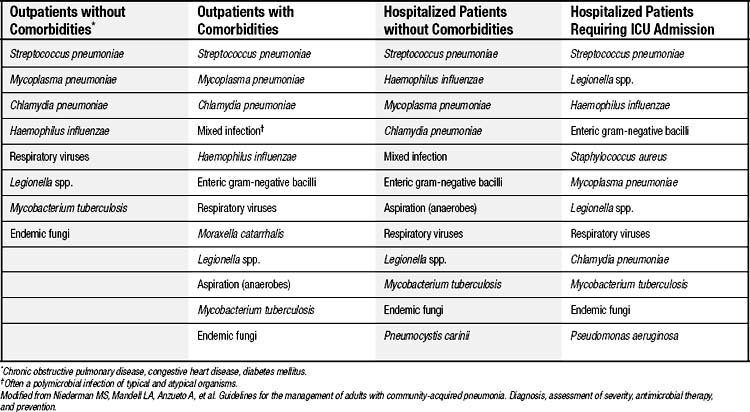
Evaluation with chest radiography should be undertaken in all patients who present with symptoms and signs suggestive of pneumonia.14 Standard posterior-anterior and lateral films classically reveal a segmental (Fig. 12-1) or lobar infiltrate, often with evidence of a parapneumonic effusion. Chest films also will help distinguish pneumonia from other disorders that may present with similar symptoms and can help assess the severity (e.g., multilobar involvement) of the illness. Contributing or causative factors, such as bronchial obstruction, lung abscess, and tuberculosis, can also be seen. The addition of computed tomography may occasionally be helpful in difficult cases, but there are few data to support its routine use in the initial evaluation of patients with CAP.
Routine laboratory studies in ambulatory patients are rarely necessary but may occasionally steer the clinician toward hospital admission in equivocal cases. Laboratory studies in hospitalized patients should include complete blood count, electrolyte values and glucose concentration, renal and liver function tests, and assessment of oxygen saturation. Patients aged 15 to 54 years should undergo testing for human immunodeficiency virus (HIV) infection with informed consent.14 Tests used to identify an etiologic agent (including blood cultures and sputum Gram stain and culture) should be performed for all patients admitted with pneumonia, although some have questioned the utility of blood cultures in low-risk patients.15 Viral cultures have not been shown to be useful in the initial evaluation of patients with CAP and should not be routinely performed,16 although some improvement in the diagnostic yield has been demonstrated with real-time polymerase chain reaction analysis.17 Every attempt should be made to obtain these culture specimens before initiation of therapy, but not at the expense of unnecessary delays in treatment. Culture data should be correlated with the Gram stain result before narrowing antibiotic therapy. Significant pleural effusions should be tapped and sent for laboratory and culture studies. Routine serologic and cold agglutinin testing is generally not recommended in the initial evaluation of patients with CAP,3 but it may prove useful in select cases. This recommendation is based on the low failure rate seen with empiric therapy for patients treated in the outpatient setting. A pneumococcal urinary antigen assay recently approved by the Food and Drug Administration may be used to augment the standard diagnostic methods,18–20 with the advantage of rapid results similar to the Gram stain. Sensitivity and specificity rates of 80% to 90% have been reported. Use of similar technology to detect pneumococcal antigen in sputum has also been reported.21
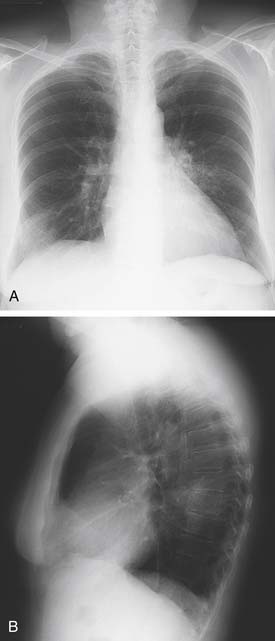
Patients with pneumonia due to Mycoplasma species often present with signs and symptoms reminiscent of a mild viral illness, with a more gradual onset and less intense nature. These infections, like other “atypical” infections, occur more frequently in younger patients. Headache, malaise, sore throat, low-grade fever, and rhinorrhea are common. Cough, when it is present, is frequently nonproductive and spasmodic in character. Extrapulmonary manifestations may predominate, with rash, arthralgias, and neurologic abnormalities present. The physical examination of the chest may yield minimal findings, although a wheeze and crackles can sometimes be appreciated. Radiologic findings include nodular or peribronchial infiltrates, an interstitial pattern of disease (Fig. 12-2), and occasional consolidation. Pleural effusions occur frequently. Although the white cell count is often normal, hemolytic anemia can be associated with a positive Coombs test result. Serologic diagnosis may be obtained through measurement of IgM or IgG titer by a complement fixation test.
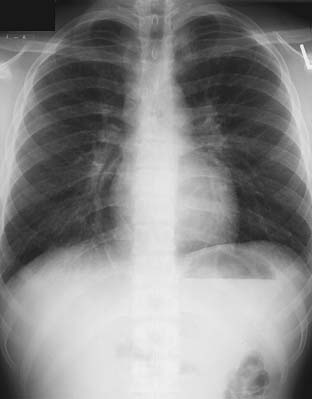
Figure 12–2 Posteroanterior chest radiograph demonstrating a fine interstitial pattern due to Mycoplasma pneumonia.
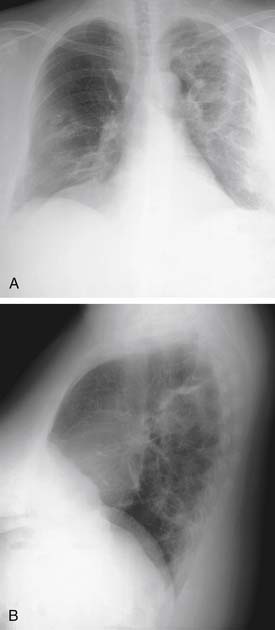
Pneumonia due to Legionella (Legionella pneumophila) remains problematic since the initial recognized outbreak of the disease at an American Legion convention in Philadelphia in 1976. The organism is identified in 1% to 5% of patients hospitalized with pneumonia, with considerable variations in detection because of geographic patterns and difficulties in accurate diagnosis. Culture may still be the best way of identifying Legionella, along with a urinary antigen assay.22 However, the urinary antigen assay can remain positive months after the acute infection. Symptoms suggestive of pneumonia predominate (high fever, chills, dyspnea, cough), but extrapulmonary manifestations (gastrointestinal complaints, malaise, myalgias) are not uncommon. Radiographs usually reveal either patchy or diffuse interstitial infiltrates and occasionally consolidation (Fig. 12-3).
Determination of Severity
One of the key decisions made in the initial evaluation of CAP, beyond establishing a presumptive diagnosis, pertains to the need for inpatient therapy. Many of the patients afflicted with CAP are elderly with significant comorbidities, for whom the progressive pulmonary infection is clearly life-threatening. On the other hand, improper admission for CAP therapy taxes the health-care system billions of dollars annually, as noted before. In the United States, less than 20% of patients with CAP are admitted for inpatient therapy but account for more than 90% of the costs related to the disease treatment.5 As a result, various guidelines have been developed to assist clinicians in the assessment of severity of illness at presentation. One such system, the Pneumonia Severity Index (PSI), was validated with data from almost 2300 patients in the Pneumonia Patient Outcomes Research Team (PORT) cohort study.23 On the basis of clinical parameters obtained at initial presentation, a risk score or class is determined (Fig. 12-4). Patients with a risk class of I to III had a low mortality rate (<1%) and were thought to be at low risk, thus amenable to outpatient management. Patients with a risk class of IV or V had higher mortality rates (9.3 and 27.0, respectfully) and were thought to be appropriate candidates for hospitalization. The value of the PSI has since been confirmed in multiple studies,24–29 and it is endorsed within the guidelines set forth by the American Thoracic Society/Infectious Diseases Society of America.3 These guidelines emphasize a multistep process in determining the initial site of treatment based on (1) assessment of factors that compromise the safety of home care, (2) PSI score, and (3) clinical judgment. The PSI has been criticized, though, because of the relative complexity of the assessment required.
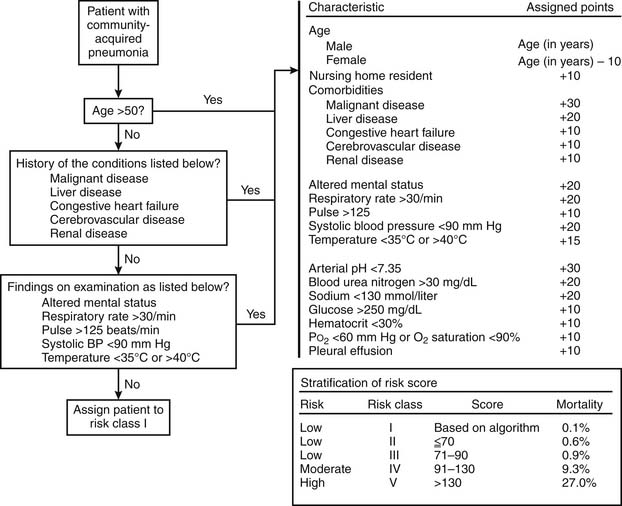
Figure 12–4 The Pneumonia Severity Index.
(Modified from Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia.)
In contrast, the British Thoracic Society has adopted an alternative prediction rule for risk stratification in the setting of CAP, termed CURB-65.30 The CURB-65 uses five prognostic variables: confusion; blood urea nitrogen concentration (>20 mg/dL); respiratory rate (>30 breaths per minute); low blood pressure (systolic, <90 mm Hg; diastolic, <60 mm Hg); and age older than 65 years. Patients with scores of 0 or 1 were thought to be appropriate for outpatient care; those with scores of 2 or 3 should be considered for admission, perhaps to the ICU; and scores of 4 or 5 mandated ICU care. A simplified version of CURB-65, termed CRB-65, drops the blood urea nitrogen measurement and thus is applicable to the outpatient office setting.31
The PSI and the CURB-65 have been compared in a variety of studies,24,32,33 and both have been found to have strengths and weaknesses. The PSI is particularly good at identifying patients with a low risk of mortality but tends to underestimate severity of illness in some populations of patients. Alternatively, the CURB-65 more accurately identifies those patients with severe CAP and at high risk of death. It has been proposed that the two assessment tools may be thought of as complementary rather than competitive tests.34
Treatment
The treatment of CAP at initial presentation is outlined in Figure 12-5, as described by the American Thoracic Society/Infectious Diseases Society of America, stratified by the presence or absence of modifying factors, comorbidities, and site of initial treatment.3 Data from published reports support the use of the guidelines, leading to better outcomes.10,35–37 The timing of therapy is important, with the first dose of antibiotic given 4 to 8 hours after initial presentation. Prompt administration of therapy has been associated with improved survival.38 The use of the guidelines facilitates prompt delivery of appropriate therapy. Further, although it is desirable to narrow the spectrum of administered antibiotic agents, this is not possible in up to 50% of cases because of a lack of an identifiable organism. In addition, a significant proportion of cases represent mixed polymicrobial infections. The use of these guidelines employing broad-spectrum therapy allows appropriate treatment in these patients.
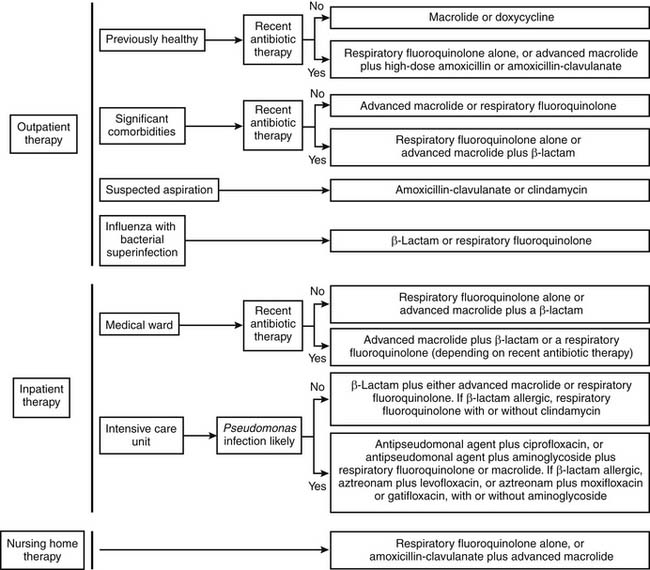
Figure 12–5 Empiric therapy for community-acquired pneumonia.
(Modified from Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults; and Mandell LA, Bartlett JG, Dowell SF, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults.)
Successful response to therapy in a patient with CAP usually follows a predictable course. Stabilization of clinical parameters often occurs by 72 hours, with gradual improvement noted thereafter. The severity of the presenting illness and the underlying disease understandably play a role in the response to therapy. The febrile response and leukocytosis typically resolve by day 4 to 5, with the radiologic findings lagging behind. In fact, the initial radiographic abnormalities may actually worsen before improvement is documented. Only 15% of chest films are clear at discharge, and many take 2 to 3 months to return to normal. With a successful response to treatment, consideration should be given to conversion to oral therapy. The American Thoracic Society describes four criteria that should be met before switching to oral therapy: improvement in cough and dyspnea; afebrile (<100°F) at two consecutive assessments 8 hours apart; resolving leukocytosis; and a functioning gastrointestinal tract with oral intake.4 Obviously, some leeway may be allowed in these criteria, given the clinical situation. Compliance can be an issue with oral therapy, and drug dosing schedules and side effects should be taken into account in selecting an oral regimen.39
Two adjunctive therapies have been recently studied for their effects on outcome in patients with severe CAP. In a retrospective analysis, the administration of activated protein C to a subset of patients in the PROWESS study with CAP was associated with a drop in mortality rates.40 Further studies, including prospective randomized trials, remain pending. In at least one randomized study, the continuous infusion of corticosteroids in patients with CAP was associated with lower mortality rates, shorter length of ICU stay, and shorter duration of mechanical ventilation.41 No increase in complications was associated with the use of steroids in this population of patients.
Two serum markers, C-reactive protein and procalcitonin, have been studied to assess response to CAP therapy, to determine outcome, and to guide duration of therapy. Of the two, procalcitonin seems the most promising. Procalcitonin is the precursor to calcitonin and has no hormonal effects. Serum levels of procalcitonin rise in the setting of acute bacterial infections, stimulated by cytokines, microbial toxins, and the cell-mediated immune response. One study determined that higher serum levels of procalcitonin, within 24 hours of admission to the hospital, were associated with higher PSI scores and a greater risk of mortality.42 Other investigators have found procalcitonin levels useful in predicting outcome and guiding duration of therapy.43–45
Hospital-Acquired (Nosocomial) Pneumonia
Hospital-acquired pneumonia refers to pulmonary infection after at least 48 to 72 hours of admission to an acute care facility or in intubated patients. The latter situation is termed ventilator-associated pneumonia. Hospital-acquired pneumonia is the second most common nosocomial infection and has the highest mortality,46–48 with crude mortality rates of 30% previously reported.49 Risk factors for nosocomial pneumonia have been described and include the extremes of age, chronic lung disease, previous abdominal or thoracic surgery, endotracheal intubation, and duration of mechanical ventilation.50
Two factors predispose patients to nosocomial pneumonia: the aerodigestive tract is colonized with bacteria, and the contaminated secretions are aspirated into the lower respiratory system.51 Several of the preventive strategies described in the following are aimed at interruption of these two processes.
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia is a nosocomial pulmonary infection developing in patients receiving mechanical ventilation. Ventilator-associated pneumonia that occurs early, within 48 to 72 hours after intubation, is typically due to bacteria responsive to antibiotic therapy (e.g., pansensitive S. aureus, H. influenzae, and S. pneumoniae) and frequently results from aspiration complicating the intubation process. In contrast, late-onset ventilator-associated pneumonia is often caused by antibiotic-resistant organisms (e.g., methicillin-resistant S. aureus, Pseudomonas aeruginosa, Acinetobacter and Enterobacter species).52 Risk factors for ventilator-associated pneumonia include the duration of mechanical ventilation, age older than 70 years, H2 or antacid therapy, chronic lung disease, depressed consciousness, need for reintubation, and gastric aspiration.46,52–55 The incidence of ventilator-associated pneumonia is significantly higher in surgical ICUs as opposed to medical ICUs, according to the Centers for Disease Control and Prevention.56
There is some controversy in the literature about optimal diagnosis of ventilator-associated pneumonia.57 To date, there seems to be little evidence supporting the use of invasive (bronchoscopic) techniques for diagnosis compared with quantitative tracheobronchial aspirates, with no difference in subsequent mortality, length of hospital stay, or duration of mechanical ventilation.58–62 Bronchoscopy may be of use in patients who fail to respond to initial therapy.63 The treatment of ventilator-associated pneumonia consists of general supportive care and the administration of empiric broad-spectrum antibiotics, guided by the severity of the illness.46,64 The adequacy of initial antibiotic therapy appears to be the most important determinant of outcome.65,66 Numerous studies have examined preventive measures to limit the development of ventilator-associated pneumonia. Semirecumbent positioning, sucralfate instead of H2 antagonists for stress ulcer prophylaxis, and selective digestive tract decontamination were found to have the strongest support in the literature.67
Health Care–Associated Pneumonia
Health care–associated pneumonia refers to pneumonia that occurs in a nonhospitalized patient with extensive health-care contacts. Examples of such contacts include outpatient intravenous therapy, hospitalization or treatment at a hospital or dialysis unit within the past 30 days, and residence in a nursing home or other long-term care facility. The recommendations for treatment mirror those suggested for hospital-acquired pneumonia.46
Aspiration Pneumonia
Aspiration refers to the inhalation of oropharyngeal or gastric contents into the tracheobronchial tree. This usually results in either a pneumonitis caused by a chemical injury from contact with sterile, acidic gastric contents or a pneumonia due to inhalation of infected material. Rarely, aspiration can lead to airway obstruction, lung abscess, and other findings. Aspiration pneumonia is a common problem, accounting for 5% to 15% of CAP, and is frequently seen in nursing home residents, those with swallowing disorders, and patients with altered mental states. It is also a recognized complication of general anesthesia, occurring in about 1 in 3000 operations and accounting for 10% to 30% of deaths related to anesthesia.68
The acute lung injury or pneumonitis from gastric aspiration, termed Mendelson’s syndrome,69 appears directly related to the acidity and volume of aspirated material. In general, a pH below 2.5 and a volume in excess of 0.3 mL/kg (about 21 mL in a 70-kg adult) are required to initiate lung injury. The injury follows a biphasic pattern; the initial insult is caused by direct damage of the acid on the delicate capillary and alveolar cells, followed several hours later by the rapid accumulation of inflammatory cells and mediators, resulting in a local and systemic inflammatory response. Progression to acute respiratory distress syndrome and multiorgan system failure may ensue. Because the gastric contents are sterile due to the acidity, infection is usually not an initial feature in these cases but may become prominent later in the course of the illness with secondary infection of the damaged lung parenchyma. Efforts to modify the gastric pH may lead to gastric colonization with gram-negative bacteria as well as with other organisms, leading to infection early after the aspiration episode. Treatment is largely supportive in nature. Particularly if the aspiration is witnessed, bronchoscopy is indicated to remove any residual fluid or debris from the airway. The use of corticosteroids in the treatment of aspiration pneumonitis has not been shown to be of benefit. Although it is common practice to initiate antibiotic therapy in these patients, this often overtreats simple pneumonitis, which may resolve with supportive measures only. Further, the use of antibiotics early may encourage the development of resistant organisms. Antibiotic therapy should be considered early in those thought to have gastric colonization and in those patients who do not improve within 48 hours of the inciting event, with evidence of ongoing lung injury, infection, or infiltrate.70 Empiric coverage with broad-spectrum agents is recommended.
Aspiration pneumonia commonly arises from inhalation of colonized oropharyngeal bacteria, causing the signs, symptoms, and radiographic changes of pneumonia. About half of normal adults aspirate while asleep,71 and for infection to occur, the normal protective mechanisms must either fail or be overwhelmed by the volume or bacterial burden of the aspirate. In supine patients, the dependent lung segments (posterior aspects of upper lobes, superior segments of lower lobes) are affected most frequently. In upright individuals, the basilar segments, particularly on the right side, are at greatest risk of infection. The aspiration episode in patients developing pneumonia is frequently not observed, and the diagnosis is derived from the clinical picture and characteristic radiographic changes in individuals known to be at risk. Treatment with antimicrobial therapy is appropriate. Use of antibiotics with anaerobic coverage is probably overdone but appropriate in select cases in which severe periodontal disease, alcoholism, and other disorders predominate. In hospitalized patients, coverage of gram-negative bacteria is essential.
Bronchiectasis
Bronchiectasis was first described in 1819 by Laennec. It is defined by the permanent dilation of the bronchi,72 caused by a recurrent process of transmural infection and inflammation. The disease process is characterized by the pathologic or radiographic appearance of the airways. Cylindrical or tubular bronchiectasis results in dilated, slightly tapered airways; varicose bronchiectasis resembles the chronic venous state of the same name, with areas of dilation and constriction; and in saccular or cystic bronchiectasis, progressive dilation of the airway can end in saclike, cystic structures resembling a cluster of grapes. The cylindrical changes are frequently seen with tuberculosis infections; the saccular or cystic type is more common after obstruction or bacterial infection. Thick, mucoid secretions are often pooled in the dilated airways, causing a chronic inflammatory state involving the airway walls. The lung parenchyma distal to the dilated, ectatic airways is often damaged as well, with fibrosis and emphysematous changes present. The accompanying bronchial arteries and lymph nodes are engorged and hypertrophied as well. The left lower lobe is the area most frequently involved, followed by the lingula and right middle lobe.
The causes and disease states associated with bronchiectasis are listed in Table 12-2. The common pathway for all these disorders is recurrent, transmural infection of the bronchial wall. Bacterial infections, particularly those involving potentially necrotizing agents such as S. aureus, P. aeruginosa, S. pneumoniae, and various anaerobes, remain important causes of bronchiectasis, particularly when there is a delay in treatment or there are factors present to prevent eradication of the infection. Bronchiectasis in patients with allergic bronchopulmonary aspergillosis is due to an immune reaction to the fungal organism, with production of inflammatory mediators and subsequent direct airway invasion by the fungus. Viral infections can lead to bronchiectatic airways both through direct infection and through a reduction in host defenses. This latter theme is common in the pathophysiologic process of bronchiectasis. Primary ciliary dyskinesia and various immune deficiencies, such as hypogammaglobulinemia, are examples of congenital disorders in which there is impairment in host defense mechanisms. Cystic fibrosis is another important cause of bronchiectasis, with predilection for the upper lobes. On occasion, the appearance of bronchiectasis in middle age will be the presenting symptom in patients with milder forms of cystic fibrosis. Several autoimmune disorders, such as rheumatoid arthritis and inflammatory bowel disease, have been linked to recurrent pulmonary infections and the development of bronchiectasis.
Table 12–2 Conditions Causing or Associated with Bronchiectasis
| Infection |
| Bacterial |
| Mycobacterial |
| Aspergillus |
| Viral (including HIV infection) |
| Congenital conditions |
| Primary ciliary dyskinesia |
| α1-Antitrypsin deficiency |
| Cystic fibrosis |
| Tracheobronchomegaly (Mounier-Kuhn syndrome) |
| Cartilage deficiency (Williams-Campbell syndrome) |
| Pulmonary sequestration |
| Marfan syndrome |
| Immunodeficiency |
| Hypogammaglobulinemia |
| Secondary to disease states or therapy |
| Sequelae of toxic inhalation or aspiration |
| Chlorine |
| Foreign body |
| Rheumatic conditions |
| Rheumatoid arthritis |
| Systemic lupus erythematosus |
| Sjögren’s syndrome |
| Relapsing polychondritis |
| Inflammatory bowel disease |
Modified from Barker AF. Bronchiectasis.
Both focal and diffuse forms of bronchiectasis are seen. The focal variety is often associated with an isolated abnormality causing relative or complete bronchial obstruction. An aspirated foreign body, slow-growing tumor, and broncholith are examples. Rarely, bronchial compression (as seen with middle lobe syndrome) or angulation of the bronchus (after surgical lobectomy) produces obstruction leading to recurrent infection and the development of localized disease. Bronchiectasis due to postinfectious causes is more likely to be localized, whereas disease due to congenital deficiencies is more likely to be diffuse. A study of stable patients with bronchiectasis revealed a 64% incidence of colonization with what were termed potential pathogenic microorganisms. The most frequently isolated potential pathogenic microorganisms were H. influenzae, Pseudomonas species, and S. pneumoniae. Risk factors for colonization by potential pathogenic microorganisms included diagnosis of bronchiectasis before 14 years of age, an FEV1 of 80% predicted, and the presence of varicose or saccular bronchiectasis.73
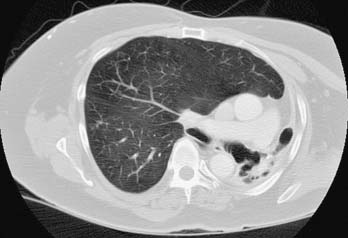
The radiographic findings in bronchiectasis are understandably important in establishing the diagnosis. Standard radiographs are abnormal in the clear majority of cases, demonstrating focal areas of consolidation, atelectasis, evidence of thickened bronchi (best noted as ring shadows when seen on end), and, in advanced cases, delineation of the dilated, cystic changes in the airway (Fig. 12-6). Computed tomography, particularly with high-resolution images, is more sensitive and specific for the diagnosis of bronchiectasis. Evidence of airway dilation, changes consistent with saccules or varicosities of the airway, and lack of airway tapering toward the periphery are all consistent with bronchiectasis.74 Upper lobe involvement suggests the diagnosis of cystic fibrosis or allergic bronchopulmonary aspergillosis; middle lobe and lingular disease is more typical of environmental mycobacterial infection, such as Mycobacterium avium complex; and lower lobe predominance suggests bacterial involvement.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree