History of Defibrillation and Cardioversion
Early in the 20th century, the Consolidated Edison Electrical Company of New York, concerned by accidental electrocutions of its line workers, supported research on the mechanisms and treatment of electrical accidents. Investigators at Johns Hopkins Hospital developed techniques of defibrillation—the termination of ventricular fibrillation—by an electrical shock in the 1930s.1 The first human defibrillation in the operating room was performed by Claude Beck in 1947.2 Transchest defibrillation using alternating current became a clinical reality when introduced by Paul Zoll in 1956,3 and direct current defibrillation was pioneered by Bernard Lown in 1962.4 The work of Zoll and Lown, in combination with the description of closed-chest cardiac massage by Jude and colleagues in 1960,5 has formed the foundation of cardiopulmonary resuscitation from cardiac arrest for 50 years.
Lown used a damped sinusoidal waveform, which—at the usually encountered human transthoracic impedance (60-90 ohms)—was effectively monophasic. In the Soviet Union, Gurvich described an underdamped sinusoidal waveform that was effectively biphasic6; this waveform was not used in the West.
More recently, truncated exponential biphasic waveforms have become the standard for transchest defibrillation; this is discussed further on.
Mechanisms of Defibrillation and Cardioversion
How does an electric shock terminate a cardiac arrhythmia? There are three principal hypotheses. The critical mass hypothesis suggests that some proportion of the myocardium (not necessarily all) must be depolarized, so that the remaining muscle is inadequate to maintain the arrhythmia.7 The upper limit of vulnerability hypothesis argues that a sufficient current density throughout the ventricle must be achieved lest fibrillation be reinitiated by a subthreshold current density.8 Jones’ group9 hypothesized that defibrillating shocks must achieve an extension of refractoriness in sufficient myocardium to terminate VF. These concepts are not mutually exclusive; all may be applicable. Whether they also apply to the atrial myocardium for the termination of atrial fibrillation by electrical shock is not known. More organized arrhythmias, such as ventricular tachycardia and atrial flutter, terminate with lower energy than VF and atrial fibrillation,10 likely because only regional depolarization in the path of an advancing wavefront is required.
Should Defibrillation Be Performed Immediately Upon Discovery of VF, or Should It Be Preceded by a Period of CPR?
VF, a lethal arrhythmia, requires prompt termination as a lifesaving maneuver. For many years the American Heart Association encouraged immediate defibrillation of a victim of VF upon the arrival of personnel equipped with a defibrillator. However, recent investigations by Cobb et al11 and Wik et al12 have shown that if the initial application of shock is delayed, a brief period of cardiopulmonary resuscitation (CPR; ventilation, closed-chest compression) before the first shock will favorably enhance outcome. (A third clinical trial, by Jacobs et al,13 did not find that a period of CPR before defibrillation facilitates resuscitation.) These observations led Weisfeldt and Becker14 to propose a three-phase model of VF-induced cardiac arrest: (1) The electrical phase consists of the first 4 minutes of VF. Shocks administered during this period have a high likelihood of achieving VF termination and resumption of spontaneous circulation. (2) The circulatory phase lasts from 4 to 10 minutes of VF. Defibrillation attempts during this period should be delayed in favor of a period of 1 to 3 minutes of CPR, including epinephrine or vasopressin, achieve at least some myocardial perfusion, thereby creating a more favorable milieu for defibrillation. (3) The metabolic phase begins after 10 minutes of VF. In this phase, changes in myocardial metabolism after prolonged VF require aggressive and invasive measures for reversal, such as cardiopulmonary bypass and/or hypothermia. Shocks given during this period without such preparatory measures are likely to result in pulseless electrical activity or asystole—conditions associated with a very low likelihood of survival.
The phases of VF/cardiac arrest outlined above are time-based. Could we achieve a more sophisticated insight into the myocardial milieu and thereby determine whether immediate electrical shock or preshock pharmacologic or other resuscitative maneuvers should be employed in each particular case? Such insight might be afforded by a detailed analysis of the electrocardiographic VF signal itself. Experimental and clinical studies have shown that changes in VF frequency and amplitude occur over time. Such changes can be modulated pharmacologically, may correlate with coronary perfusion pressure (the difference between aortic and right atrial pressure during the relaxation phase of closed-chest compression), and may predict the response to a defibrillating shock and the success of the resuscitation efforts.15–18 With better understanding of the VF signal and its relationship to the state of the myocardium, the optimal timing of the electrical shock could be guided by a microprocessor-based analysis of the VF signal integrated into the defibrillator, which would instantly instruct the operator on whether to deliver a shock or use other supportive measures, such as continuing CPR and administering vasopressors. Defibrillators using such sophisticated techniques of VF analysis are now commercially available.
Who Should Be Cardioverted from Atrial Fibrillation/Atrial Flutter, and When Should This Be Performed?
Sinus rhythm improves cardiac performance, especially in patients with mitral stenosis, left ventricular hypertrophy (aortic stenosis, hypertension, idiopathic hypertrophic subaortic stenosis), and/or diminished myocardial reserve (congestive heart failure, myocardial ischemia, and infarction). The coordinated atrial contraction of sinus rhythm improves ventricular filling, and cardiac rate is usually slower. Patients with these conditions are thus candidates for elective cardioversion. Urgent cardioversion may be required for patients with atrial or ventricular arrhythmias who are hypotensive and/or in pulmonary edema.
In some cases, treatment of an underlying or causative condition may restore sinus rhythm without the necessity of electrical cardioversion. Common causes of atrial arrhythmias include hyperthyroidism, pulmonary embolism, congestive heart failure, and mitral stenosis. Postoperative cardiac patients frequently experience transient rhythm disturbances that may spontaneously revert to sinus rhythm.
Important factors that determine the immediate and long-term success of cardioversion of atrial arrhythmias include the duration of the arrhythmia, the extent of atrial fibrosis, and the size of the left atrium. High success rates have been reported for cardioversion of atrial fibrillation and atrial flutter, especially when the new biphasic waveforms are used. High transthoracic impedance (TTI)—the resistance of the chest to the flow of electrical current—may reduce transcardiac current flow.19 This appears to be of less importance for the termination of atrial arrhythmias when biphasic rather than monophasic waveforms are used. The ability of biphasic defibrillators to adjust their waveform according to TTI probably accounts for this. Sequential defibrillation modestly and consistently decreases TTI when either monophasic or biphasic waveforms are used.20,21
Thromboembolism
There is a significant risk of thromboembolism after cardioversion. Three factors contribute to this risk: (l) If there is a preexisting thrombus in the fibrillating atrium (especially likely in the left atrial appendage), the electrical shock and/or the resumption of atrial contraction may dislodge the thrombus. (2) The shock itself may have thrombogenic effects. (3) With prolonged atrial fibrillation an atrial myopathy develops, which results in a delayed return to normal atrial contraction after cardioversion. To prevent thromboembolism, therapeutic anticoagulation (international normalized ratio of 2.0-3.0) for 3 weeks before cardioversion and 4 weeks afterward has traditionally been recommended if the patient has been in atrial fibrillation for more than 48 hours (often the exact duration of the arrhythmia is unknown, especially if the patient is asymptomatic).
The risk of thromboembolism associated with atrial fibrillation and cardioversion is higher in patients with mitral stenosis, a large left atrium from any cause, chronic atrial fibrillation of long duration, previous thromboembolic events, diabetes, or hypertension. Although transthoracic echocardiography is able to image the left atrial cavity well, it is usually unsatisfactory for visualization of the left atrial appendage, the site of most atrial thrombi. However, the newer technique of transesophageal echocardiography (TEE) images the left atrial appendage well and is highly sensitive to the presence of thrombi. Manning and colleagues22 reported no embolic events during the cardioversion of atrial fibrillation when transesophageal echocardiography showed that no thrombi were present in the atrial appendage and the patient received intravenous heparin for 2 days before cardioversion. This approach is known as TEE-guided cardioversion.23 If a thrombus in the left atrial appendage is seen by TEE, cardioversion should be delayed, and anticoagulation for 3 weeks should be undertaken. Some clinicians repeat the TEE to ensure that the thrombus has lysed. Although thromboembolism after cardioversion of atrial flutter is less common, it has been reported, as have conditions associated with thromboembolism, such as left atrial “smoke” (spontaneous ultrasound contrast) on transesophageal echocardiography.23,24 Thus anticoagulation before cardioversion of atrial flutter of more than short duration should be undertaken, similar to atrial fibrillation.25
At present, TEE and cardioversion are generally carried out as two separate procedures, both requiring conscious sedation or anesthesia. Recently the two procedures have been combined by adding an electrode to the external surface of the transesophageal echo probe. Another electrode is placed on the anterior chest wall using a self-adhesive electrode pad. This allows a cardioverting DC shock to be delivered, using the esophageal-chest pathway. Because the esophageal electrode is close to the heart and the pathway is shortened, less energy (typically 20-50 J) is required to terminate atrial fibrillation using this esophageal cardioversion technique. Initial clinical experience with this combined TEE/cardioversion approach has been reported.26
Whether the traditional or TEE-guided anticoagulation scheme is used, it is considered mandatory to maintain anticoagulation for at least 4 weeks after cardioversion, because in the absence of anticoagulation, thrombi may form after cardioversion and embolism may occur despite a negative TEE precardioversion.21-25 Patients with paroxysmal atrial fibrillation or those considered at high risk of recurrence of atrial fibrillation after cardioversion may require permanent anticoagulation. Antiarrhythmic drugs such as amiodarone or flecainide may facilitate cardioversion and maintenance of sinus rhythm after cardioversion. Digitalis-toxic rhythms should not be cardioverted, as the enhanced automaticity of such arrhythmias combined with the shock could result in ventricular fibrillation or ventricular tachycardia.27
Techniques of Cardioversion and Defibrillation
Because the electrical current passing across the thorax causes a painful tetanic contraction, elective cardioversion in this author’s opinion should be performed under general anesthesia; conscious sedation is often inadequate, with the patient experiencing and remembering severe discomfort. Bag-valve ventilation without endotracheal intubation is usually sufficient, but the presence of an anesthesiologist facilitates rapid endotracheal intubation if this becomes necessary.
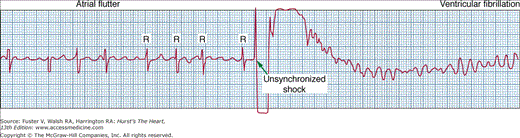
It is essential to synchronize the electrical discharge on the R wave of the QRS complex; if the shock falls in the vulnerable period of the cardiac cycle, VF may be induced (Fig. 46–1). This is the most frequent serious complication of elective cardioversion of atrial arrhythmias and usually results from the operator’s failure to enable properly the synchronizing device or to verify that the R wave of the ECG lead chosen is sufficiently tall to be recognized by the synchronizer. Recognition of the R wave of ventricular tachycardia is sometimes difficult owing to the morphology of the arrhythmia. If the patient is hemodynamically unstable owing to rapid ventricular tachycardia, unsynchronized shocks may be necessary; if VF results from the first shock, an immediate second shock is administered to terminate the VF. The operator must be prepared, in such circumstances, to quickly disable the synchronizing setting; if this is not done the defibrillator will not identify an R wave (which is not present in VF) and will not deliver a shock.
Figure 46–1
A complication of cardioversion: induction of ventricular fibrillation. The ventricular arrhythmia occurred because the operator failed to enable the synchronizer, resulting in inadvertent delivery of the shock on the vulnerable T wave instead of the intended delivery on the R wave. This complication is preventable by enabling the synchronizer and checking that it is properly functioning before shock delivery. From Kerber RE. Transchest cardioversion: optimal techniques. In: Tacker WA, ed. Defibrillation of the Heart: ICDs, AEDs and Manual. St. Louis, MO: Mosby-Year Book; 1994: Chapter 7. Reproduced with permission from the author.