The association between incomplete revascularization (IR) and long-term mortality after stenting in the era of drug-eluting stents is not well understood. In the present study, we test the hypothesis that IR is associated with a greater risk of long-term (5-year) mortality after stenting for multivessel coronary disease. Using data from the Percutaneous Coronary Intervention Reporting System of New York State, 21,767 patients with multivessel disease who underwent stenting during October 2003 to December 2005 were identified. Complete revascularization (CR) was achieved in 6,844 patients (31.4%), and 14,923 patients (68.6%) were incompletely revascularized. The CR and IR patients were propensity matched on a 1:1 ratio on the number of diseased vessels, the presence of total occlusion, type of stents, and the probability of achieving CR estimated using a logistic model with established risk factors as independent variables. Patients were followed for vital status until December 31, 2008 using the National Death Index. Differences in survival between the matched CR and IR patients were compared. Among the 6,511 pairs of propensity-matched patients, the 5-year survival rate for IR was lower compared with CR (79.3% vs 81.4%, p = 0.004), and the risk of death during follow-up was 16% greater for IR compared with CR (hazard ratio 1.16, 95% confidence interval 1.06 to 1.27, p = 0.001). In addition, subgroup analyses demonstrated that the association between IR and long-term mortality was not dependent on major patient risk factors. In conclusion, IR is associated with an increased risk of long-term mortality after stenting for multivessel disease in the era of drug-eluting stents.
Previous studies on the impact of incomplete revascularization (IR) on outcomes for percutaneous coronary intervention (PCI) with implantation of stents were primarily based on data from the era of bare-metal stents (BMS). More recent studies using data from the era of drug-eluting stents (DES) have in general found that IR was associated with a greater risk of major cardiac adverse events (death, repeat revascularization, or myocardial infarction) after PCI. However, findings regarding the impact of IR on mortality have been inconsistent across studies. Some studies have found that IR is not associated with an increased risk of mortality after PCI, but other studies, including our previous study, reported that IR is associated with a greater risk of mortality. Moreover, the lengths of follow-up in the previous studies using the data from the DES era were usually not >3 years. Therefore, there is a need for more studies that investigate the association between IR and mortality in longer follow-up periods. In the present study, we examined the impact of IR on mortality for a longer follow-up period (≤5 years) using data from a larger patient population. We hypothesized that IR was associated with a greater risk of long-term (5-year) mortality after stenting for multivessel disease in the DES era.
Methods
The primary database used in the present study is the Percutaneous Coronary Intervention Reporting System (PCIRS) of the New York State. The PCIRS is maintained by the New York State Department of Health and contains detailed information on every PCI procedure performed in nonfederal hospitals in the State since early 1990s. Data collected by the PCIRS relevant to the present study include patient demographic variables, patients’ preprocedural risk factors, detailed information about lesions in coronary arteries, including pre- and postprocedural stenosis for each lesion, the procedure performed, and the intracoronary devices used for each lesion, major postprocedural complications, and the disposition at discharge. To ensure the completeness of the PCIRS database, data submitted by hospitals to the PCIRS are periodically matched to the hospital discharge database of New York State. To ensure the accuracy of the data reported by the hospitals, data validation is conducted regularly by the review agent of the New York State Department of Health by reviewing samples of medical records for cases reported to the PCIRS. The PCIRS was used to identify the study population and to track repeat PCI procedures after the index stenting procedure using patient identifiers including social security numbers, dates of birth, admission, procedure, and discharge.
Data from the Cardiac Surgery Reporting System (CSRS) New York State were also used in the study. The CSRS registers all major cardiac surgical procedures performed in nonfederal hospitals in the State. It uses similar data collection and auditing methods to that used in the PCIRS. In short, patient demographic variables, risk factors before surgery, procedural information, complications, and discharge status are reported to the CSRS by hospitals.
In addition, the National Death Index maintained by the National Center for Health Statistics was used in the present study. The National Death Index collects all death certificate records in the United States, and it was used to ascertain the vital status of the study population in the follow-up period after hospital discharge.
The PCIRS and CSRS were used to identify the study population. The inclusion criteria were (1) patients had undergone stenting procedures with DES or BMS during October 1, 2003 to December 31, 2005 in New York, (2) had lesions with stenosis of ≥70% in ≥2 major epicardial arteries (left anterior descending artery and major diagonals, left circumflex artery and large marginal branches, and right coronary artery and right posterior descending artery), (3) had no lesions with stenosis ≥50% in the left main coronary artery, (4) had no history of PCI or coronary artery bypass graft surgery before the index stenting procedures, (5) had no acute myocardial infarction within 24 hours before the index procedures, and (6) had not undergone coronary artery bypass graft surgery in the index admission or within 30 days of discharge of the index procedure. A total of 21,767 patients who underwent stenting met the inclusion criteria and were included in the present study.
The completeness of revascularization of stenting was determined by the degree of postprocedural stenosis in all lesions with preprocedural stenosis ≥70% in major epicardial coronary vessels. Complete revascularization (CR) was defined when the postprocedural stenosis in each of the lesions was reduced to <50% in the index hospitalization or within 30 days in staged PCI procedures after discharge from the index hospitalization before the occurrence of a new myocardial infarction. When CR was not achieved after the stenting procedure in the index admission or within 30 days of discharge, the revascularization was defined as IR.
The outcome variable is all-cause mortality after the index stenting procedure. Patients’ vital statuses were tracked by matching to the National Death Index on social security number, date of birth, and gender. The follow-up of vital statuses ended on December 31, 2008. For a patient who died before the end of 2008, the length of follow-up was calculated as the time interval from the date of the index procedure to the date of death. Otherwise, the follow-up ended on December 31, 2008, and the survival time was censored. The median length of follow-up was 3.9 years with an interquartile range of 3.4 to 4.6 years.
The framework of the analysis is a propensity-matched survival analysis. First, the IR and CR patient groups were compared with respect to the distributions of baseline characteristics including demographics, preprocedural risk factors, New York State PCI risk score for in-hospital mortality, and type of stents (DES or BMS) using the Student’s t test for continuous variables and Pearson’s chi-square test for categorical variables.
A logistic regression model was then fit to predict the probability of receiving CR using all available baseline risk factors. Using this propensity model, a propensity score (the log-odds of probability of receiving CR) was obtained for each patient. Next, we attempted to match each CR patient to an IR patient on the number of diseased vessels, the presence of total occlusion, the type of stents (DES only, BMS only, or both), and the value of the propensity score. The matching caliper for the propensity score was set as 0.6 × SD of its distribution. The standardized differences in means for continuous variables and in proportions for categorical variables between the matched IR and CR patients were calculated to examine how well the baseline risk factors in the 2 groups were balanced after matching.
The data from the propensity-matched patients were then used to compare the Kaplan-Meier survival curves for IR and CR by the log-rank test. In addition, the hazard ratio for death for IR compared with CR was obtained by fitting a Cox proportional hazards model. The analysis was repeated for each type of IR defined by the number of IR vessels, that is, 1-vessel IR and at least 2-vessel IR.
Subgroup analysis was then conducted to test the significance of interactions between IR and preselected baseline risk factors such as age, ejection fraction, a history of myocardial infarction and congestive heart failure, diabetes, proximal left anterior descending artery disease, proximal vessel disease (stenosis ≥70% in proximal left anterior descending artery, right coronary, or left circumflex artery), the presence of a total occlusion, renal failure, and New York State PCI risk score for in-hospital mortality (≥8 vs <8). This analysis consists of fitting a Cox proportional hazards model for each of these risk factors. In each model, IR, the risk factor of interest, and their interaction term were included as independent variables, along with other significant predictors of survival (p <0.05) determined by a backward selection approach. The adjusted hazard ratio for death for IR versus CR was then calculated for each level of the risk factor of interest, and the significance of the interaction term was examined at the α = 0.05 level.
Because most patients only received DES, a sensitivity analysis was conducted by restricting the analysis to the DES patients to evaluate whether the impact of IR on long-term mortality in such patients is consistent with that observed in the entire study population. All statistical analyses were conducted in SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
In the study population of 21,767 patients, 18,374 (84.4%) received only DES, and 1,815 (8.3%) received only BMS. During the index hospitalization or within 30 days after discharge, 6,844 patients (31.4%) were completely revascularized, and 14,923 patients (68.6%) were incompletely revascularized. There were variations in the prevalence of IR across providers. The interquartile ranges of prevalence of IR were 61.1% to 76.4% across hospitals and 60.7% to 81.1% across operators.
IR was associated with older age, being non-Hispanic black or Hispanic, lower values of ejection fraction, 3-vessel disease, presence of total occlusion, history of a number of diseases such as myocardial infarction, cerebrovascular disease, peripheral arterial disease, congestive heart failure, diabetes, or renal failure, slightly greater New York State PCI risk score for in-hospital mortality, and greater likelihood of implantation of BMS only ( Table 1 ).
| Variable | Incomplete Revascularization, n = 14,923 (%) | Complete Revascularization, n = 6,844 (%) | p |
|---|---|---|---|
| Age (yrs) | <0.001 | ||
| <50 | 1,308 (8.8) | 707 (10.3) | |
| 50–59 | 3,235 (21.7) | 1,656 (24.2) | |
| 60–69 | 4,160 (27.9) | 1,951 (28.5) | |
| 70–79 | 4,042 (27.1) | 1,747 (25.5) | |
| ≥80 | 2,178 (14.6) | 783 (11.4) | |
| Gender | 0.57 | ||
| Women | 4,848 (32.5) | 2,250 (32.9) | |
| Men | 10,075 (67.5) | 4,594 (67.1) | |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic white | 10,605 (71.1) | 5,373 (78.5) | |
| Non-Hispanic black | 1,577 (10.6) | 518 (7.6) | |
| Hispanic | 1,598 (10.7) | 534 (7.8) | |
| Other | 1,143 (7.7) | 419 (6.1) | |
| Body surface area (m 2 ), mean (SD) | 2.01 (0.27) | 2.02 (0.26) | <0.001 |
| Body mass index group (kg/m 2 ) | 0.09 | ||
| <18.5 | 146 (1.0) | 60 (0.9) | |
| 18.5–24.99 | 3,378 (22.6) | 1,467 (21.4) | |
| 25–29.99 | 5,888 (39.5) | 2,686 (39.2) | |
| ≥30 | 5,511 (36.9) | 2,631 (38.4) | |
| Ejection fraction | <0.001 | ||
| <20% | 884 (5.9) | 378 (5.5) | |
| 20–29% | 605 (4.1) | 142 (2.1) | |
| 30–39% | 1,120 (7.5) | 323 (4.7) | |
| ≥40% | 12,296 (82.4) | 5,994 (87.6) | |
| Missing | 18 (0.1) | 7 (0.1) | |
| Number of diseased vessels | <0.001 | ||
| 2 | 10,080 (67.5) | 6,115 (89.3) | |
| 3 | 4,843 (32.5) | 729 (10.7) | |
| Presence of total occlusion | 5,967 (40.0) | 684 (10.0) | <0.001 |
| Previous myocardial infarction | <0.001 | ||
| 1–7 d | 2,942 (19.7) | 1,309 (19.1) | |
| 8–20 d | 408 (2.7) | 116 (1.7) | |
| ≥21 d | 2,181 (14.6) | 529 (7.7) | |
| No myocardial infarction before procedures | 9,392 (62.9) | 4,890 (71.4) | |
| Cerebrovascular disease | 1,275 (8.5) | 489 (7.1) | <0.001 |
| Peripheral arterial disease | 1,273 (8.5) | 373 (5.5) | <0.001 |
| Hemodynamic state | 0.27 | ||
| Stable | 14,887 (99.8) | 6,832 (99.8) | |
| Unstable | 30 (0.2) | 12 (0.2) | |
| Shock | 6 (0.0) | 0 (0.0) | |
| Congestive heart failure | <0.001 | ||
| This admission | 1,266 (8.5) | 392 (5.7) | |
| Before this admission | 388 (2.6) | 121 (1.8) | |
| None | 13,269 (88.9) | 6,331 (92.5) | |
| Malignant ventricular arrhythmia | 77 (0.5) | 22 (0.3) | 0.05 |
| Chronic obstructive pulmonary disease | 981 (6.6) | 430 (6.3) | 0.42 |
| Diabetes requiring medication | 5,106 (34.2) | 1,947 (28.4) | <0.001 |
| Renal failure | <0.001 | ||
| Requiring dialysis | 352 (2.4) | 130 (1.9) | |
| Creatinine >2.5 mg/dl (220 μmol/L) | 226 (1.5) | 51 (0.7) | |
| No renal failure | 14,345 (96.1) | 6,663 (97.4) | |
| New York State PCI risk score for in-hospital mortality, mean (SD) | 3.9 (3.1) | 3.3 (2.8) | <0.001 |
| Type of stent | <0.001 | ||
| BMS only | 1,449 (9.7) | 366 (5.3) | |
| DES only | 12,687 (85.0) | 5,687 (83.1) | |
| Both BMS and DES | 787 (5.3) | 791 (11.6) |
A total of 6,511 CR patients (95.1%) were propensity matched to IR patients using a 1:1 ratio. The baseline risk factors were well balanced between the matched IR and CR groups with standardized differences in the means and prevalences of such risk factors not >2.7% (all p values >0.05; Table 2 ).
| Variable | Incomplete Revascularization, n = 6,511 (%) | Complete Revascularization, n = 6,511 (%) |
|---|---|---|
| Age (yrs) | ||
| <50 | 636 (9.8) | 674 (10.4) |
| 50–59 | 1,524 (23.4) | 1,591 (24.4) |
| 60–69 | 1,838 (28.2) | 1,850 (28.4) |
| 70–79 | 1,712 (26.3) | 1,652 (25.4) |
| ≥80 | 801 (12.3) | 744 (11.4) |
| Gender | ||
| Women | 2,191 (33.7) | 2,162 (33.2) |
| Men | 4,320 (66.3) | 4,349 (66.8) |
| Race/Ethnicity | ||
| Non-Hispanic white | 5,030 (77.3) | 5,098 (78.3) |
| Non-Hispanic black | 508 (7.8) | 492 (7.6) |
| Hispanic | 546 (8.4) | 514 (7.9) |
| Other | 427 (6.6) | 407 (6.3) |
| Body surface area (m 2 ), mean (SD) | 2.02 (0.27) | 2.02 (0.26) |
| Body mass index group (kg/m 2 ) | ||
| <18.5 | 56 (0.9) | 60 (0.9) |
| 18.5–24.99 | 1,390 (21.3) | 1,393 (21.4) |
| 25–29.99 | 2,602 (40.0) | 2,560 (39.3) |
| ≥30 | 2,463 (37.8) | 2,498 (38.4) |
| Ejection fraction | ||
| <20% | 353 (5.4) | 349 (5.4) |
| 20–29% | 133 (2.0) | 130 (2.0) |
| 30–39% | 313 (4.8) | 308 (4.7) |
| ≥40% | 5,707 (87.7) | 5,717 (87.8) |
| Missing | 5 (0.1) | 7 (0.1) |
| Number of diseased vessels | ||
| 2 | 5,782 (88.8) | 5,782 (88.8) |
| 3 | 729 (11.2) | 729 (11.2) |
| Presence of total occlusion | 684 (10.5) | 684 (10.5) |
| Previous myocardial infarction | ||
| 1–7 d | 1,261 (19.4) | 1,247 (19.2) |
| 8–20 d | 122 (1.9) | 109 (1.7) |
| ≥21 d | 501 (7.7) | 506 (7.8) |
| No myocardial infarction before procedures | 4,627 (71.1) | 4,649 (71.4) |
| Cerebrovascular disease | 460 (7.1) | 461 (7.1) |
| Peripheral arterial disease | 371 (5.7) | 344 (5.3) |
| Hemodynamic state | ||
| Stable | 6,503 (99.9) | 6,501 (99.8) |
| Unstable or shock | 8 (0.1) | 10 (0.2) |
| Congestive heart failure | ||
| This admission | 382 (5.9) | 370 (5.7) |
| Before this admission | 123 (1.9) | 111 (1.7) |
| None | 6,006 (92.2) | 6,030 (92.6) |
| Malignant ventricular arrhythmia | 20 (0.3) | 19 (0.3) |
| Chronic obstructive pulmonary disease | 405 (6.2) | 409 (6.3) |
| Diabetes requiring medication | 1,922 (29.5) | 1,858 (28.5) |
| Renal failure | ||
| Requiring dialysis | 125 (1.9) | 119 (1.8) |
| Creatinine >2.5 mg/dl (220 μmol/L) | 51 (0.8) | 47 (0.7) |
| No renal failure | 6,335 (97.3) | 6,345 (97.5) |
| New York State PCI risk score for in-hospital mortality, mean (SD) | 3.4 (2.7) | 3.3 (2.8) |
| Type of stent | ||
| BMS only | 366 (5.6) | 366 (5.6) |
| DES only | 5,687 (87.3) | 5,687 (87.3) |
| Both BMS and DES | 458 (7.0) | 458 (7.0) |
During the follow-up until December 31, 2008, 1,045 IR and 888 CR patients died among the 6,511 pairs ( Table 3 ). IR was associated with 16% greater risk of death (hazard ratio 1.16, 95% confidence interval (CI) 1.06 to 1.27, p = 0.001). The 5-year Kaplan-Meier survival rate was 2.1% lower in the IR group (79.3% vs 81.4%, p = 0.004; Figure 1 ).
| Patient Group | No. of Cases | No. of Deaths | Hazard Ratio (95% CI) | p |
|---|---|---|---|---|
| Incomplete revascularization | 6,511 | 1,045 | 1.16 (1.06–1.27) | 0.001 |
| Complete revascularization | 6,511 | 888 | Reference | |
| Subgroups of incomplete revascularization | ||||
| 1 incompletely revascularized vessel | 5,413 | 846 | 1.13 (1.02–1.25) | 0.01 |
| Matched completely revascularized patients | 5,413 | 734 | Reference | |
| Multiple incompletely revascularized vessels | 1,098 | 199 | 1.27 (1.03–1.57) | 0.03 |
| Matched completely revascularized patients | 1,098 | 154 | Reference | |
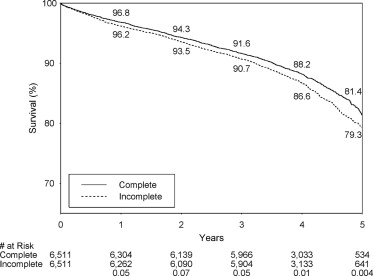
Each type of IR was associated with a significantly greater risk of death than CR, and the respective hazard ratios for the death for 1-vessel and multivessel IR were 1.13 (p = 0.01) and 1.27 (p = 0.03; Table 3 ). The respective 5-year survival rates for 1-vessel IR and matched CR patients were 79.8% and 81.4% (p = 0.03), and the respective 5-year survival rates for multivessel IR and matched CR patients were 77.0% and 81.0% (p = 0.04).
Of the 20 subgroups examined, IR was associated with significantly greater risk of death than CR in 13 subgroups (p <0.05; Table 4 ). In addition, none of the interactions between IR and the selected risk factors was statistically significant (all p values ≥0.09). Thus, there was no evidence that the association between IR and long-term mortality was dependent on these risk factors.



