In-hospital infections (IHI) are one of the most common and serious problems after invasive procedures. Transcatheter aortic valve implantation (TAVI) is an increasingly used alternative to surgery in patients with severe symptomatic aortic stenosis. The aim of this study was to determine the incidence, origin, risk factors, and clinical outcomes of IHI after TAVI. A total of 303 consecutive patients with severe aortic stenosis who underwent transfemoral TAVI were included and followed during a median time of 21 months. We examined the occurrence, types, origin, and timing of infections during hospital stay as well as short- and long-term clinical outcomes according to the occurrence of IHI. A total of 51 patients (17%; 62 infectious episodes) experienced IHI after TAVI. Respiratory and urinary tract infections were the most frequent type of infections (44% and 34%, respectively), followed by surgical site infection (8%) and bloodstream infection (5%). Positive cultures were obtained in 74% of the samples, of which 65% were gram-negative bacilli. Modifiable factors such as bleeding (p = 0.005) and length of coronary care unit stay (p <0.001) were independently associated with an increased infection risk. Patients with IHI had a longer hospital stay (14 vs 6 days, p <0.001), an increased mortality (hazard ratio 2.48, 95% CI 1.45 to 4.23) and readmission rate (hazard ratio 2.0, 95% CI 1.27 to 3.14) during the follow-up. In conclusion, IHI is a frequent complication after TAVI with a significant impact on short- and long-term clinical outcomes. The most important risk factors associated with the development of this complication were modifiable periprocedural aspects. These results underline the importance to implement specific preventive strategies to reduce in-hospital–acquired infections after TAVI.
In-hospital infections (IHI) are one of the most common complications that may occur after medical and surgical admissions, with estimates ranging from 3.7% to 4.0%. Surgical site infections (SSIs), bloodstream infections, urinary tract infections, and respiratory complications (including pneumonia) are the most frequent IHI in patients who undergo any type of surgery or are admitted to an intensive/coronary care unit (CCU). Importantly, any IHI significantly impacted length of hospital stay, costs, and clinical outcomes. Approximately, 1/3 of hospital-acquired infections are preventable and several measures, and less invasive procedures have been successfully proved to reduce IHI rates. Previous studies of patients undergoing cardiac surgery and percutaneous cardiac interventions have reported an incidence of IHI ≈5% and ≈0.64%, respectively. Transcatheter aortic valve implantation (TAVI) is currently the standard percutaneous treatment for severe aortic stenosis in symptomatic patients at high surgical risk. The burden of co-morbidities in patients undergoing TAVI and the invasive periprocedural techniques confers a high likelihood of IHI in such patients. In fact, IHI and systemic inflammatory response syndrome has been reported as a major cause and an independent predictor of mortality in TAVI population. Identifying the timing, causes, and predictors of IHI are thus fundamental to implement appropriate preventive measures. However, data on IHI after TAVI are scarce, especially regarding modifiable factors to reduce IHI. More importantly, no data exist on the impact of IHI on long-term mortality and readmission after TAVI. The objectives of this study were to determine the incidence, origin, and factors associated with IHI and its clinical impact after TAVI.
Methods
A total of 308 consecutive patients with symptomatic severe aortic stenosis who underwent transfemoral TAVI in a single center were evaluated. Of these, 5 patients who died during the procedure were excluded, leading to a final population of 303 patients. The indication for TAVI was decided in a weekly heart team meeting with clinical and interventional cardiologists and cardiac surgeons. All TAVI procedures were performed in the catheterization laboratory through a fully percutaneous transfemoral approach using balloon and self-expanding valves, as previously described. Contralateral femoral artery and vein were obtained to monitor the procedure and perform rapid ventricular pacing and, if not needed, removed the same day of the procedure after normalization of the activated clotting time. No other central catheters were routinely placed. General anesthesia (GA) or monitored deep sedation was determined by TAVI anesthesiologist and interventional cardiologist according to patients’ characteristics and preprocedural status. All patients with GA were extubated at the end of the procedure whenever possible. Conversion to GA in patients with deep sedation was performed if required due to intraprocedural complications. Prophylactic antibiotic therapy (cefazoline or vancomicin 1 g in case of penicillin allergy) was administered 1 hour before the procedure and followed by 2 additional doses, 8 hours apart to complete a 3-dose regimen according to the local practice guidelines. Patients stayed a minimum of 24 hours in the CCU after the procedure. Vital signs, including temperature, were monitored every 8 hours, and a blood sample was taken before the procedure and at least once daily up to hospital discharge. The length of CCU/hospital stay was defined as the interval between the day of the procedure and the day of CCU/hospital discharge or in-hospital death. Hospital stay before the procedure was also recorded in patients with urgent or emergent indication (elective patients were admitted the day before the intervention). In-hospital and follow-up data were prospectively entered in a dedicated database. Clinical outcomes were defined according to Valve Academic Research Consortium criteria. All patients signed informed consent forms before the procedure, and all studies were performed in accordance with the local ethics committee.
IHI was determined by the combination of symptoms reported by the patient, signs detected during the physical examination in the intensive cardiac care unit or cardiology ward, and by the results provided by laboratory tests. Cultures were obtained in all patients, and the causative agent was established by the microbiology laboratory. The specific types of infection were defined according to the criteria of the Center for Disease Control and Prevention and were classified as follows: urinary tract infection, SSI, bloodstream infection, pneumonia, upper and lower respiratory tract infection, and skin and soft tissue infection, among others. The infection date was defined as the first day of symptoms or positive culture results. Time-to-infection was calculated as the time between TAVI procedure and the date of infection. Clinical follow-up was carried out in a prescheduled outpatient TAVI clinic at 1, 6 and 12 months after procedure and yearly thereafter. Clinical major adverse events and hospital readmissions were entered prospectively in the dedicated database, with a median follow-up of 21.4 (interquartile range 10.0 to 38.0) months. No patients were lost to follow-up.
All data were analyzed with the Stata Statistical Software: Release 14 (StataCorp. 2015; StataCorp LP, College Station, Texas). Categorical variables are expressed as n (percentage) and continuous variables as mean (SD) or median (interquartile range 25th to 75th percentile) according to their distribution. Assessment of normality for continuous data was performed using the Shapiro–Wilk test. Comparison of qualitative variables was performed with the chi-square or Fisher’s exact test. Quantitative variables with a normal or nonnormal distribution were analyzed with a 2-sided Student t or Wilcoxon rank-sum or median test, respectively. Anesthesia type (general vs deep sedation) was entered in the model as an intention-to-treat analysis. Variables with a p value <0.10 in the univariate analysis were entered into a Cox regression analysis to determine the independent predictors of IHI and death. Freedom from mortality and readmission curves was calculated using the Kaplan–Meier method, and comparison was obtained with the log-rank test.
Results
The baseline characteristics, procedure details, and in-hospital complications for the overall study population and for patients with and without IHI are listed in Tables 1 and 2 . The procedure was elective in 240 patients (79%), and GA was used in 1/3. A total of 28 patients with an initial strategy of deep sedation were converted to GA due to intraprocedural hemodynamic instability or complications during the procedure.
| Overall (n = 303) | In-hospital infection | p Value | ||
|---|---|---|---|---|
| Yes (n = 51) | No (n = 252) | |||
| Variable | ||||
| Age (years) | 84 [79-87] | 83 [80-87] | 84 [79-87] | 0.750 |
| Woman | 191 (63%) | 30 (59%) | 161 (64%) | 0.494 |
| Weight (kg) | 70 [61-80] | 72 [60-82] | 70 [62-80] | 0.434 |
| Body mass index (kg/m 2 ) | 27.3 [24.9-30.5] | 27.3 [24.8-31.2] | 27.3 [24.9-30.4] | 0.920 |
| Frailty | 58 (19%) | 9 (18%) | 49 (19%) | 0.785 |
| New York Heart Association class III or IV | 229 (78%) | 40 (80%) | 189 (77%) | 0.693 |
| Diabetes mellitus | 107 (35%) | 21 (41%) | 86 (34%) | 0.337 |
| On insulin | 38 (13%) | 7 (14%) | 31 (12%) | 0.611 |
| Hypertension | 249 (82%) | 44 (86%) | 205 (81%) | 0.402 |
| Coronary artery disease | 145 (48%) | 24 (47%) | 121 (48%) | 0.901 |
| Prior cardiac surgery | 35 (12%) | 3 (6%) | 32 (13%) | 0.165 |
| Atrial fibrillation | 119 (39%) | 18 (35%) | 101 (40%) | 0.523 |
| Chronic obstructive pulmonary disease | 69 (23%) | 13 (25%) | 56 (22%) | 0.612 |
| Previous cerebrovascular accident | 35 (12%) | 7 (14%) | 28 (11%) | 0.594 |
| Peripheral vascular disease | 20 (7%) | 4 (8%) | 16 (6%) | 0.446 |
| eGFR (mL/min) | 61.4 [47.5-75.8] | 61.9 [47.1-75.5] | 61.3 [47.5-76.2] | 0.978 |
| Hemoglobin (g/dL) | 12.13 (±1.69) | 12.11 (±1.59) | 12.14 (±1.72) | 0.935 |
| EuroSCORE II | 3.69 [2.59-6.04] | 4.42 [2.71-7.23] | 3.6 [2.59-5.62] | 0.062 |
| Echocardiographic variables | ||||
| Left ventricular ejection fraction (%) | 60 [46-70] | 60 [40-73] | 60 [46-70] | 0.715 |
| Mean aortic gradient (mmHg) | 46 [39-55] | 46 [42-53] | 46 [39-55] | 0.415 |
| Aortic valve area (cm 2 ) | 0.5 [0.4-0.7] | 0.5 [0.4-0.7] | 0.5 [0.4-0.7] | 0.636 |
| Overall (n = 303) | In-hospital Infection | p Value | ||
|---|---|---|---|---|
| Yes (n = 51) | No (n = 252) | |||
| Procedural characteristics | ||||
| Prior Balloon valvuloplasty | 188 (65%) | 29 (62%) | 159 (66%) | 0.549 |
| Prosthesis type | 0.586 | |||
| Balloon-expandable | 204 (67%) | 36 (71%) | 168 (67%) | |
| Self-expandable | 99 (33%) | 15 (29%) | 84 (33%) | |
| Prosthesis size (mm) | ||||
| 23 | 106 (35%) | 18 (35%) | 88 (35%) | 0.979 |
| 26 | 137 (46%) | 24 (47%) | 113 (46%) | 0.845 |
| 29 | 56 (19%) | 9 (18%) | 47 (19%) | 0.828 |
| Urgent procedure | 63 (21%) | 16 (31%) | 47 (19%) | 0.041 |
| Anesthesia | 0.041 | |||
| General | 104 (35%) | 24 (47%) | 80 (32%) | |
| Deep sedation | 196 (65%) | 27 (53%) | 169 (68%) | |
| Procedure time (minutes) | 120 [90-150] | 120 [90-155] | 120 [90-145] | 0.115 |
| In-hospital complications | ||||
| In-hospital mortality | 22 (7%) | 7 (14%) | 15 (6%) | 0.071 |
| Stroke | 7 (2%) | 3 (6%) | 4 (2%) | 0.096 |
| Vascular complications | ||||
| Major | 37 (12%) | 11 (22%) | 26 (10%) | 0.027 |
| Requiring surgical access repair | 22 (7%) | 9 (18%) | 13 (5%) | 0.002 |
| Bleeding complications | ||||
| Life-threatening | 29 (10%) | 13 (26%) | 16 (7%) | <0.001 |
| Major | 25 (8%) | 4 (8%) | 21 (9%) | 0.957 |
| Acute kidney injury 2-3 | 16 (5%) | 6 (12%) | 10 (5%) | 0.035 |
| New permanent pacemaker implantation | 49 (16%) | 8 (16%) | 41 (16%) | 0.918 |
| Lengh of coronary care unit stay (days) | 1 [1-2] | 3 [1-6] | 1 [1-2] | <0.001 |
| Lengh of hospital stay (days) | 7 [6-11] | 14 [8-27] | 6 [5.5-8] | <0.001 |
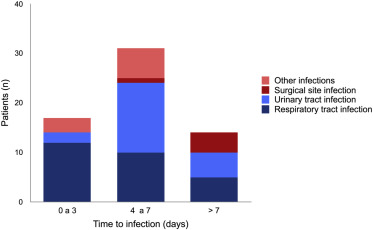
IHI after TAVI was identified in 51 patients (17%) with a total of 62 infectious episodes (8 patients had multiple infections). Infections types are presented in Table 3 . Median time from TAVI to first infection was 5 days (2 to 8 days). A detailed summary of the time to IHI, CCU, and hospital stay length is depicted in Supplementary Figure 1 . Infections occurring within the first 72 hours were mainly respiratory (71%, p = 0.016), whereas late (>72 hours) IHI were predominantly urinary or had a surgical site origin (64%, p = 0.002; Figure 1 ). Cultures were positive in 74% of the samples, of which 2/3 were gram-negative bacilli ( Supplemental Table 1 ). The independent predictors of IHI are listed in Table 4 .
| Type of infection | Patients (n = 51) | Events (n = 62) | Days from TAVI to first infection | ||
|---|---|---|---|---|---|
| Median | Min | Max | |||
| Respiratory tract infection (total) | 20 (39%) | 27 (44%) | 3.5 | 0 | 23 |
| Pneumonia | 10 (29%) | 14 (23%) | 4 | 0 | 15 |
| Lower respiratory tract infection, other than pneumonia | 6 (12%) | 9 (15%) | 7 | 0 | 23 |
| Upper respiratory tract infection | 4 (8%) | 4 (7%) | 1.5 | 0 | 2 |
| Urinary tract infection | 15 (29%) | 21 (34%) | 6 | 2 | 14 |
| Surgical site infection | 4 (8%) | 5 (8%) | 12 | 9 | 19 |
| Access site infection | 1 (2%) | 2 (3%) | 5.5 | 5 | 6 |
| Bloodstream infection | 1 (2%) | 3 (5%) | 7 | 2 | 7 |
| Intravascular cannulation site infection | 1 (2%) | 3 (5%) | 5 | 5 | 5 |
| Skin and soft tissue infection | 1 (2%) | 1 (2%) | 2 | 2 | 2 |
| Univariable analysis OR (95% CI) | p value | Multivariable analysis OR (95% CI) | p value | |
|---|---|---|---|---|
| Urgent indication | 1.99 (1.02-3.90) | 0.044 | 1.97 (0.96-4.29) | 0.087 |
| General Anesthesia | 1.88 (1.02-3.46) | 0.043 | 1.82 (0.89-3.69) | 0.100 |
| Life threatening bleeding | 5.03 (2.24-11.31) | <0.001 | 3.67 (1.48-9.09) | 0.005 |
| Acute kidney injury 2-3 | 3.23 (1.12-9.32) | 0.030 | ||
| Length of CCU stay ∗ | 1.28 (1.16-1.42) | <0.001 | 1.24 (1.12-1.37) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


