Introduction
Remarkable advancements have been made over the last several decades in our understanding of the basic mechanisms and treatments related to cardiovascular diseases. This has led to dramatic improvements in public health. Between 1960 and 2000, for example, overall life expectancy for newborns in the United States increased by nearly seven years with approximately 70% of these gains in survival resulting specifically from lower rates of cardiovascular death.1 For coronary artery disease (CAD) alone, age-adjusted mortality fell by more than 50% during the past two decades.2 Similar patterns of improvements in survival have also been noted for patients with heart failure, despite an overall rising prevalence of this condition due to aging populations.3,4
Importantly, many of these benefits have been attributed to the discovery of evidence-based therapies in cardiology. Aspirin, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors and statins can reduce the risk of death after acute myocardial infarction (AMI) by 20 – 35%.5,6 The timely use of acute reperfusion therapy in ST elevation myocardial infarction lowers mortality by an additional 25%.7 In heart failure, ACE inhibitors, angiotensin receptor blockers (ARB) and aldosterone blockers have all been linked to significant improvements in quality of life as well as lower rates of hospital admissions and mortality in patients with impaired left ventricular (LV) systolic function.8 Similar advances have been noted in atrial fibrillation with anticoagulation therapy,9 acute stroke management with fibrinolytic therapy,10 and in the primary and secondary prevention of cardiovascular events using aspirin, statins and lifestyle modifications like smoking cessation counseling.11
Development of new therapies has been the traditional role of large national biomedical enterprises like the National Institutes of Health (NIH), as well as the pharmaceutical and device industries. Over the last several decades, this framework has been enormously successful in expanding the “evidence” behind much of what we know. However, it has clearly fallen short in its ability to guide these same scientific breakthroughs into routine clinical practice. Despite established data supporting their use, long-term adherence rates to evidence-based therapies such as aspirin, beta-blockers and statins in patients with CAD remain low in community-based practices across the United States, at between 45% and 70%.12 For heart failure, long-term adherence rates for ACE inhibitors may be as low as 20%.12 In fact, some estimates suggest that it takes on average 17 years for new therapies to be adopted into widespread clinical practice.13 Simply increasing the use of existing evidence-based therapies in cardiology would have profound implications for the healthcare system and enhance the public health as much as new discoveries.
Practice gaps, knowledge translation and quality improvement science
The practice gaps between “what we know” and “what we do” have been well documented and widely recognized by policy makers and providers, although the reasons for them are less certain.14 Moreover, these practice gaps are not limited to any single country or healthcare system but have been shown in a variety of regions and settings.15 Strategies to address these challenges were introduced years ago but gained much more momentum during the 1990s, evolving from approaches that relied on the passive diffusion of knowledge to recent efforts to incorporate information technology and systems engineering into a transformation of the healthcare delivery system. Yet despite these advances, substantial practice gaps remain in medicine and these have been difficult to overcome. In its landmark report in 2001, the Institute of Medicine (IOM) referred to these practice gaps as the “quality chasm” and called for renewed efforts to develop better systematic approaches for bringing evidence to the bedside.16
Accordingly, this chapter focuses on strategies for improving the implementation of evidence-based cardiology based on research in knowledge translation. Knowledge translation has been described variably but a frequently cited definition is the one proposed by the Canadian Institutes of Health Research:
“Knowledge translation is a dynamic and iterative process that includes synthesis, dissemination, exchange and ethically sound application of knowledge to improve the health of [populations], provide more effective health services and products and strengthen the health care system.”17
Furthermore, the scientific investigation of specific methods to promote the use of research findings in clinical practice may be considered “quality improvement” science or research.18 In different settings, similar concepts have been labeled as “knowledge transfer research” and “implementation science or research”. In this chapter, we most frequently use the terms “knowledge translation” and “quality improvement science”.
In the following sections, we discuss (1) the overall scope of the problem in practice gaps, (2) possible barriers identi-fied in translating evidence to clinical practice, and (3) spe-cific approaches that have been studied in quality improvement science to overcome these barriers. Many of the issues raised here are universal to medicine but we try to focus on examples in cardiology while still highlighting broader themes. We end the chapter by briefly discussing unique challenges faced in quality improvement science, including the growing tension between the immediate desire to improve quality and the rigor required for objectively studying its improvement.
Practice gaps: scope of the problem
Evidence-based medicine has been described as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients”.19 The application of evidence-based medicine therefore is necessarily dependent upon the existence of a mature base of knowledge that can inform providers of what treatments are appropriate (and inappropriate) in specific clinical settings. Despite the fact that persistent voids continue to remain in some key clinical areas within cardiology, no specialized field of medicine is as well developed or evidence-based. This makes cardiology ideally suited in many ways for providers to optimize their use of evidence-based approaches. Yet even in areas of cardiology with a clear base of evidence, like acute myo-cardial infarction (AMI), heart failure, atrial fibrillation and hypertension, the practice gaps between “what we know” and “what we do” remain large and extend across the entire spectrum of care from inpatient to outpatient settings (Table 6.1).
Table 6.1 Recent examples of practice gaps in cardiovascular disease
| Condition | Example |
| Coronary | Beta-blocker use: |
| artery disease |
|
| Heart failure | ACE inhibitor use:
|
| Atrial | Anticoagulation: |
| fibrillation |
|
| Hypertension | Blood pressure management:
|
Hospital setting
Practice gaps in the inpatient setting have been demonstrated for a number of disease processes in cardiology. For example, acute reperfusion therapy with either fibrinolytic therapy or primary percutaneous coronary intervention (PCI) in ST elevation myocardial infarction reduces mortality and morbidity. While the use of acute reperfusion therapy has improved over recent years, recent data suggest that worldwide, as many as a third of eligible patients still do not receive it even at centers capable of delivering both treatments.20 Furthermore, the overall effectiveness of acute reperfusion therapy is highly dependent upon its timely delivery, but both fibrinolytic therapy and primary PCI are often administered after long delays. Data from just a few years ago suggest that fewer than 50% of patients in the United States were treated with primary PCI within 90 minutes or fibrinolytic therapy within 30 minutes.21 Similar practice gaps have been noted in other countries, including those in the developing world. In India, for example, evidence-based therapies like acute reperfusion therapy, beta-blockers, ACE inhibitors (or ARBs) and statins are used in between 50% and 60% of hospitalized patients with acute coronary syndromes.22
Others have documented wide regional variation in the appropriate use of cardiac catheterization and PCI after AMI (including data that suggest both under-and overuti-lization).23,24 In a recent analysis of nearly 45 000 patients, investigators noted that regions of the United States with a higher intensity of invasive procedure utilization performed cardiac catheterization at increased rates in both appropriate (i.e. for an ACC/AHA Class I indication) and inappropriate (i.e. for an ACC/AHA Class III indication) situations.24 In addition, the use of invasive procedures was inversely correlated to the baseline risk of the patient assessed by their Global Registry of Acute Coronary Events (GRACE) risk score.
Similar patterns of practice gaps in the inpatient setting (including recent trends toward improvement) have also been demonstrated in heart failure and atrial fibrillation.25,26 For example, the initial Euro Heart Surveys found rates of ACE inhibitor and beta-blocker use of 61.8% and 36.9%, respectively, among hospitalized patients with heart failure,27 although these numbers have improved somewhat in more recent surveys.28 Among inpatients and outpatients patients with atrial fibrillation enrolled at 182 hospitals in 35 countries in the Euro Heart Survey, roughly two-thirds of eligible patients were prescribed oral antico-agulation therapy.29 Furthermore, oral anticoagulation therapy was not targeted at those patients who were the highest risk for stroke but instead depended upon non-clinical factors such as the availability of outpatient laboratory monitoring.30
Outpatient setting
In 2003, McGlynn et al published a landmark study demonstrating that just over 50% of patients received “recommended” care for a variety of healthcare conditions in the outpatient setting, using a complex survey design of 12 metropolitan areas of the United States between 1998 and 2000.12 Recommended care in this study primarily focused on processes of care that were defined using the RAND-UCLA modified Delphi method to specific identify quality indicators through expert panels. Although rates of compliance with recommended care were generally higher for CAD (68%) and heart failure (64%) than other disease-specific conditions, critical practice gaps were still noted. For example, only 45% and 61% of patients with myocardial infarction received beta-blockers and aspirin, respectively, despite the absence of clear contraindications and strong evidence of long-term benefit with both. Rates of recommended care associated with atrial fibrillation were even less and dropped below 25%. Furthermore, the investigators noted broad differences in practice gaps based on the “mechanism” required for performing recommended care, regardless of the healthcare condition. Care that was related to direct encounters with providers or medications had the highest proportion of compliance at approximately 70%, whereas counseling or education was performed in fewer than 20% of cases (Table 6.2).
Table 6.2 Adherence to outpatient quality indicators by mechanism in a random sample of US adults from 12 metropolitan areas (from McGlynn et al 200312)
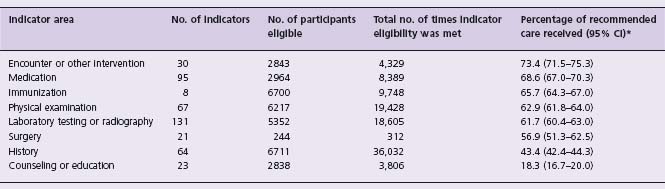
* CI denotes confidence interval. All pairwise differences were statistically significant at P < 0.001 except those between medication and encounter or other intervention (P = 0.02), physical examination and immunization (P = 0.001), surgery and immunization (P = 0.004), and surgery and physical examination (P = 0.05). The difference between surgery and laboratory testing or radiography was not significant (P = 0.39).
Similar findings were also noted in the international REACH registry, which enrolled nearly 68 000 patients 45 years or older with evidence of atherosclerotic disease from 5473 physician practices across 44 countries.31 Physician practices in this registry included a broad mix of clinical practices such as primary care providers and specialists; urban, rural and suburban environments; and office-based and hospital-based settings. Importantly, this study noted suboptimal adherence rates with statins (69%) and anti-platelet agents (79%) in these patients, as well as evidence for undertreatment of hypertension, with 50% of patients found to have elevated baseline blood pressures above 140/90 mmHg.
Disparities and the risk-treatment paradox
Key patterns have emerged from these studies with important clinical and policy implications. First, practice gaps that have been documented in clinical practice have consistently been largest among vulnerable populations, including the elderly, minority patients and women. These appear to be particularly evident for expensive and resource-intensive procedures, such as coronary revascu-larization and implantable cardioverter defibrillators (ICDs).32–34 The extent to which these practice gaps are due to biological factors, reduced access to care, or systematic biases in the delivery of care to vulnerable populations is unclear. A similar association between practice gaps and socioeconomic status has also been recognized, including examples from developing countries. In the CREATE registry, for example, 30-day mortality was significantly higher in poor hospitalized patients with acute coronary syndromes when compared with rich patients in 50 cities across India. Importantly, this mortality difference was largely explained by differences in rates of evidence-based therapy use. Studies such as the CREATE registry have provided invaluable insights by focusing on low- and middle-income countries where 80% of the global burden of cardiovascular disease exists. In general, there remains a paucity of data from these countries on practice gaps and additional work is urgently needed to improve our understanding of clinical practice patterns in these regions.
Second, there is evidence supporting a “risk-treatment” paradox for many evidence-based therapies. That is, those patients who may stand to benefit the most from a therapy paradoxically are the least likely to receive it. Among nearly 1500 patients in the EFFECT cohort with heart failure and LV systolic dysfunction, ACE inhibitor, ARB and beta-blocker use were inversely associated with the severity of the patient’s condition and their predicted risk of death in the upcoming year.35 After accounting for potential contraindications to therapy, low-risk patients were more likely to receive ACE inhibitors or ARBs (adjusted hazard ratio (HR), 1.6) and beta-blockers (adjusted HR, 1.8) than high-risk patients (both I < 0.001). Similarly, another population-based study from Ontario found statin use to be significantly lower among high-risk patients with established CAD or diabetes mellitus.36 Interestingly, recent data suggest that much of the risk-treatment paradox may be explained by clinical factors not typically captured in administrative data, including functional limitations and depressive symptoms.37
Barriers to knowledge translation
A number of common barriers to implementing evidence-based medicine in clinical practice have been identified. We focus here primarily on barriers related to provider-level (e.g. physician and hospital) and system-level factors. While we recognize the importance of patient-level factors in the use of evidence-based therapies, it is beyond the scope of this discussion. One simple conceptual model for understanding these barriers suggests that incorporating new therapies into clinical practice proceeds from changes in knowledge to attitudes to behavior (Fig. 6.1).38,39 This model mirrors other approaches that emphasized the progression from awareness to acceptance to adoption among providers,40 but slightly differs by noting that each step is potentially independent of others in the process. For example, behavior can be changed through incentives at the organizational-level without necessarily improving knowledge or attitudes among providers.
Figure 6.1 Barriers to use of guidelines and evidence-based medicine. (From Cabana et al,38 with permission.)
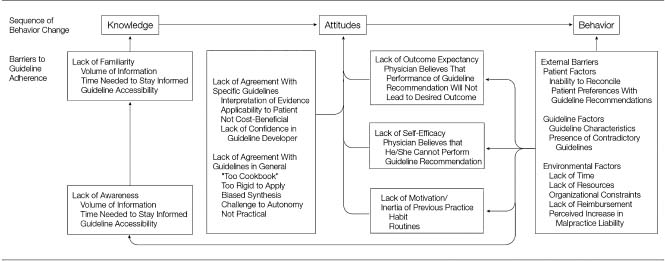
Knowledge
Improving knowledge relies on providing new information to clinicians primarily through increasing their awareness of the published literature. The enormous size and complexity of contemporary biomedical research make this a particularly challenging barrier to implementing evidence-based medicine. For example, in 2006 the top 10 clinical cardiovascular journals alone published 4065 articles, averaging 78 articles per week. It is clearly impossible for busy providers in clinical practice today to stay on top of the latest developments even in a specialized field like cardiology. An understanding of this barrier has led to the development of guidelines and evidence-based summaries to synthesize the published literature by professional organizations and other groups. In fact, it is one of the key motivations for writing this textbook.
Attitudes
Even when providers become familiar with the available evidence, recommendations may be incomplete or confusing. This can lead to barriers to changing the attitudes of providers (i.e. their acceptance of new evidence). For example, summary clinical guidelines and consensus statements from expert panels may be large, unmanageable or poorly developed.41 Conflicting recommendations also can arise if different (and sometimes competitive) organizations produce separate guidelines for the same disease process. Finally, attitudes can limit acceptance when providers feel that recommendations inadequately reflect the nuances of clinical practice, making them less applicable to their patients or their healthcare system.
Behavior
Barriers to the adoption of behavior can still exist even when providers have moved beyond awareness and acceptance of evidence. Adoption of behaviors can be particularly challenging since it requires that changes occur in the actual performance of a task at the provider or organizational level. For providers, barriers to adoption can vary from factors as ordinary as the influence of habit and routine to more complex issues that reflect lack of time or resources.42 Organizational-level factors that impact on behavior include reimbursement or payment structures for providing care as well as the availability of or access to specialized services and medications.
Finally, it is important to recognize that patients can also indirectly influence the adoption of new recommendations by providers. A perceived lack of adherence to lifestyle recommendations by patients has been described as a frequent reason by providers for inconsistent recommendations about smoking cessation.43 This also can be problematic in cases where patients often have set expectations for receiving a particular service. For example, providers may be reluctant not to pursue cardiac catheterization in an active individual with a low-risk stress test who believes it is necessary based on personal experiences (e.g. “My father had it done!”).
Multiple barriers
It is rare for a single barrier to be responsible for the limited use of an evidence-based therapy. Instead multiple barriers often contribute to poor implementation, and barriers iden-tified in one clinical environment may be less relevant for other areas or settings. For example, high out-of-pocket drug costs may be a pertinent barrier to statin use in patients with CAD, while limited availability of a high-quality program may be an important barrier for cardiac rehabilitation. In patients interested in smoking cessation, drug costs for nicotine replacement therapy and the availability of counseling programs could play important roles. Similarly, in urban areas barriers to timely access for primary PCI could be accomplished by redesigning emergency medical services to integrate new technologies like prehospital electrocardiography (ECG).44 However, large geographic distances between hospitals with and without cardiac catheterization laboratories would limit the applicability of this approach in rural healthcare systems.45
Important socioeconomic factors can also affect patterns of adoption for evidence-based therapies, especially depending upon the structure of the healthcare delivery system into which these therapies are introduced. For example, an important reason why behavior modifications such as smoking cessation, nutritional counseling and cardiac rehabilitation may be difficult to implement is that most physicians are not traditionally trained in the skills required for these activities and reimbursement is disproportionately low for them. In contrast, physicians have a greater proclivity for performing diagnostic tests and therapeutic procedures since these are what they are often trained to do best and because their reimbursement is more aligned with these services. This has a potentially perverse effect on a healthcare system by encouraging overuse of expensive procedures (even when marginally effective) and underuse of useful simple diagnostic and therapeutic approaches (even when evidence-based and cost effective).46
An important limitation of existing literature in quality improvement science is that interventions are often developed without researchers first rigorously studying what local barriers may be contributing to insufficient knowledge translation. Furthermore, even when intervention-based studies report the presence of local barriers, descriptions frequently lack adequate detail. In a large review of studies examining barriers to guideline use among physicians, Cabana and colleagues found that just half of all intervention-based studies reported more than one category of barriers in their results.38 Without a better appreciation of what barriers may have been present at baseline, it is impossible for readers to judge whether the findings associated with an intervention would be applicable in their own clinical practices.
Strategies for quality improvement
There have been a number of studies exploring different strategies for overcoming barriers to the implementation of evidence-based medicine in cardiology and improving quality. These strategies have evolved significantly over the years from simple educational interventions (i.e. one-time dissemination of educational materials) targeted at improving provider knowledge to complex and multifaceted approaches designed to leverage information and systems-based technologies (i.e. computerized order entry and reminders). On the “road map” for moving basic research into clinical practice, Dougherty and Conway have distinguished research in quality improvement science from other translational research and identified it as the third translational step (i.e. “T3”) (Fig. 6.2).47 While the first two translational steps focus primarily on moving evidence from basic science research to clinical efficacy (i.e. “T1”) and clinical effectiveness (i.e. “T2”), T3 research should be focused on understanding how evidence-based medicine can be reliably delivered to broad populations of patients across different healthcare settings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


