Chapter 85 Implantable Cardioverter-Defibrillators
Indications, Management of Complications, and Device Follow-up
Indications for Implantable Cardioverter-Defibrillator Therapy
Indications for ICD therapy, as established in 2009–2010, include patients with manifest ventricular tachyarrhythmic events (referred to as secondary prevention indications) or those at risk for sudden, symptomatic ventricular tachyarrhythmias (primary prevention indications) that could potentially result in sudden cardiac death (SCD). Updated guidelines for the use of ICDs in ventricular tachyarrhythmias were most recently published in 2008 by the expert panel from the American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) Joint Task Force.1 Of note, these guidelines, compared with earlier versions released, reflect significant changes in our understanding of the mechanisms and risks of SCD in high-risk subpopulations with coronary diseases, nonischemic heart diseases, and primary electrical diseases of the heart. Because of the overlap between primary and secondary SCD prevention indications, the recommendations have been simplified into a single list. The primary prevention indications for nonischemic cardiomyopathies have been updated to include data from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) (i.e., ischemic and nonischemic cardiomyopathies with left ventricular ejection fraction [LVEF] ≤35% and New York Heart Association [NYHA] class II to III).2 As new clinical trials continue to expand the primary prevention applications with regard to SCD, the guidelines are updated to include inherited arrhythmia syndromes and select nonischemic cardiomyopathies. In accordance with clinical practice, the primary prevention guidelines continue to expand, but the guidelines for secondary prevention remain relatively stable.
The guidelines address programming at the device’s end of life. The LVEF criteria of the current primary prevention ICD recommendations are based on the entry criteria of the randomized clinical trials that showed the benefit of such indications (e.g., Multicentre Automatic Defibrillator Trial II [MADIT-II], SCD-HeFT).2,3
These guidelines divide the recommendations into the following major classes:
The levels of evidence for these recommendations are classified as follows:
Box 85-1 summarizes the ACC/AHA/HRS 2008 practice guideline indications for ICD therapy. For convenience, the use of ICDs in the secondary and primary prevention of ventricular arrhythmias and SCD are discussed separately.
Box 85-1 Indications for Implantable Cardioverter-Defibrillator Therapy
CLASS I
CLASS IIA
CLASS IIB
CLASS III
Modified from Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol 51:1–62, 2008.
Implantable Cardioverter-Defibrillators for the Secondary Prevention of Ventricular Arrhythmias
Sustained Symptomatic Ventricular Tachycardia and Survivors of Cardiac Arrest
The first established indication for the use of the ICD was in patients who had had spontaneous sustained and symptomatic ventricular tachycardia (VT) or ventricular fibrillation (VF) or who were survivors of a cardiac arrest. In these patients, ICD devices have terminated sustained ventricular tachyarrhythmias with either anti-tachycardia pacing or shock therapy. Various studies have documented pace termination of VT in 89% to 91% of all episodes, with the residual events being converted by shock therapy.4,5 Programmed ICD therapy has also successfully converted VF in more than 98% of episodes.4,5 Failure to induce VT or VF at electrophysiological study (EPS) occurs in up to 40% of all SCD survivors.6 However, VT, VF, SCD, or all recur in 8% to 50% of these patients, and more than half of them die at the time of recurrence.6–8 In a large body of cumulative experience, the SCD rate reported with device therapy has been in the range of 1% to 2% (Figure 85-1) per year, with a cumulative incidence of less than 10% at 5 years and a significant projected survival benefit compared with untreated populations.4,5,9,10 Thus data from large multi-center, randomized trials comparing ICD therapy with various drugs for the secondary prevention of SCD in patients with VT or VF consistently indicate that device therapy is superior to guided or empiric medical therapy for these patients. In the original three trials addressing this subject—the Antiarrhythmics versus Implantable Defibrillator (AVID) trial, the Cardiac Arrest Study of Hamburg (CASH), and the Canadian Implantable Defibrillator Study (CIDS)—the total mortality rate showed an average 30% relative risk (RR) reduction in the ICD arm of the study.11–13 Individual patient groups have often varied among trials. The AVID trial excluded patients with sustained but minimally symptomatic or hemodynamically well-tolerated VT and syncope with induced sustained VT. Inclusion required the presence of hemodynamic instability or left ventricular dysfunction with VT. In contrast, CIDS included many of these subgroups. Subsequent analyses of the AVID registry data, which tracked excluded subgroups, showed that mortality rates without device therapy in patients with hemodynamically well-tolerated VT were comparable with those of other groups included in the study. In the current guidelines, all these subgroups are considered to have indications for device therapy. Also, left ventricular dysfunction as an important determinant of the benefit of defibrillation therapy in patient survival continues to be highlighted.14
In analyzing recommendations for device therapy, procedural risk and longer term complications must be factored in the equation. These and other large clinical trials have shown that the implant risk with current ICD systems is less than 0.5%.4,5,11 Table 85-1 provides the complications observed in the ICD arm in the AVID study.12 The most common clinical complication observed has been inappropriate device therapy, typically occurring in patients with atrial fibrillation (AF) with rapid ventricular response. This occurs in approximately 5% to 11% of all patients and most often in combination with appropriate device activations in the same patients in these studies.14 Careful device follow-up, operator training and experience, and refinements in technology have minimized device-related complications. A more recent report from the National Cardiovascular Data Registry (NCDR) assessed ICD implantation risks as ranging from 3.5% for electrophysiologists to 4% for thoracic surgeons and 5.8% for other implanting physicians.15 Patient outcomes improve with higher volume operators and implant centers.16 Thus, given the low perioperative mortality, limited morbidity, and overwhelming evidence for survival benefit, the latest guidelines classify cardiac arrest and sustained VT or VF as class I indications for ICD therapy.
Table 85-1 Frequency of Implantable Cardioverter-Defibrillator Complications in the First 539 Implantations in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial
| TYPE OF COMPLICATION | NO. OF PATIENTS (%) |
|---|---|
| Lead fracture | 15 (2.8) |
| Infection | 14 (2.8) |
| Bleeding/hematoma | 8 (1.5) |
| Lead dislodgment | 8 (1.5) |
| Pneumothorax with chest tube | 6 (1.1) |
| Thrombosis | 2 (0.4) |
| Cardiac perforation | 2 (0.4) |
| Generator migration/erosion | 3 (0.6) |
| Generator failure* | 4 (0.7) |
| TOTAL | 62 (11.5) |
* An additional six patients had their generators recalled.
Modified from Kron J, Herre J, Renfroe EG, et al: Lead- and device-related complications in the Antiarrhythmics Versus Implantable Defibrillator trial, Am Heart J 141:92–98, 2001.
Syncope with Inducible Sustained Ventricular Tachycardia
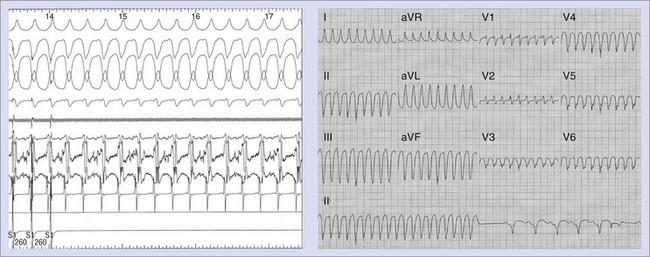
In patients with recurrent syncope, EPS may reveal inducible sustained VT, which may be the mechanism of syncope (Figure 85-2). In CIDS, patients were included with this presentation and derived similar benefits from ICD therapy as did other groups.10 These patients are considered as having symptomatic cardiac syncope and are included in the secondary prevention group. In general, they have underlying organic heart disease, often with compromised left ventricular function or regional wall motion abnormalities supporting a substrate for the tachyarrhythmia.
Specific Clinical Syndromes and Disease States with Indications for Implantable Cardioverter-Defibrillator Therapy
Coronary Artery Disease
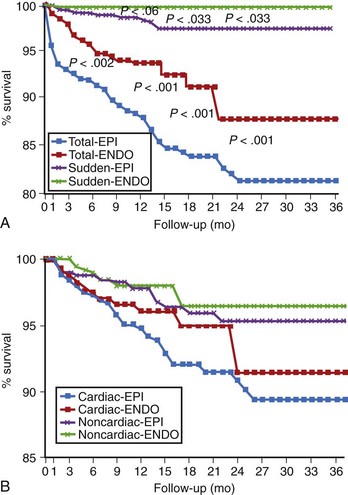
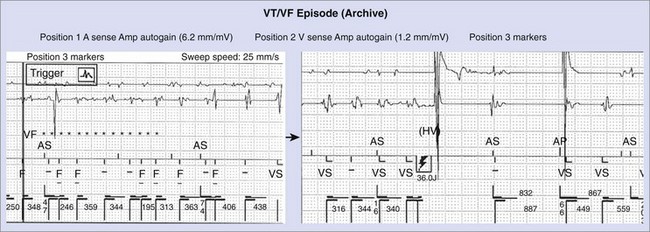
Patients with CAD comprise the majority of patients receiving ICDs in most reports.4,5,11–13 Device implantation is widely accepted as improving the outcomes of these patients. Patients with reduced left ventricular function may receive greater benefit with ICD therapy than with drug therapy.3,17 Risk stratification may be appropriate to avoid device insertion in patients with a relatively low risk of arrhythmic events. These patients can be identified by the absence of spontaneous ventricular arrhythmias, late potentials on signal-averaged electrocardiogram (ECG), T-wave alternans (TWA) on exercise testing, and absence of inducible VT or VF on programmed electrical stimulation. This is discussed in more detail in other chapters in this text. Implantation of an ICD also requires careful assessment of other comorbidities that could limit survival. Patients with advanced noncardiac disease (e.g., renal disease requiring dialysis or end-stage pulmonary disease) derive little or no survival benefit with ICD therapy. Once a decision has been made to proceed with ICD insertion in patients with significant coronary disease, ischemic status should be assessed with stress testing, coronary angiography, or both. In well-selected patients, appropriate defibrillation or cardioversion shocks have been effective in prolonging survival and have been documented in device datalogs (Figure 85-3).
To limit risk to the patient during defibrillation efficacy testing, the presence of active ischemia should be determined before proceeding with device implantation. Furthermore, optimal anti-ischemic treatment further enhances quality of life as well as survival. Ventricular function should be assessed before device implantation, although depressed function is not a contraindication to device therapy. However, uncontrolled heart failure increases the risks associated with implantation. To prevent deterioration of functional status, defibrillation threshold testing should be minimized in patients with elevated pulmonary capillary wedge pressure or severely compromised cardiac output.18
Nonsustained Ventricular Tachycardia with Coronary Artery Disease and Left Ventricular Dysfunction
This patient group is the first primary prevention category adopted as a consequence of the MADIT-I.19 This subgroup has long been recognized to have a high propensity for SCD and inducible sustained VT. In early studies, Wilber and colleagues recognized that electrophysiological provocation was capable of stratifying the risk of SCD in this population with the induction of sustained VT.20 The investigators of MADIT-I hypothesized that ICD therapy would improve survival in this high-risk population. They demonstrated a 54% reduction in the relative risk of death in these patients compared with that of conventional drug therapy such as amiodarone. The study was prematurely terminated, and the indication has now been widely adopted. It has been estimated that approximately 3% to 7% of survivors of acute myocardial infarction (MI) in different series will eventually be stratified into this subgroup. Implantation experiences have confirmed the MADIT data that virtually 50% of these patients have a sustained ventricular tachyarrhythmia after prophylactic ICD implantation within 18 months, confirming the original hypothesis.21 Cost-effectiveness analyses have been very favorable, with an estimated $27,000 per life-year saved with transvenous implantation. Recent trends with declining device costs and shorter hospitalizations in these patients are likely to further improve the cost effectiveness of this therapy.
Syncope in Coronary Artery Disease
Patients with CAD and syncope of undetermined etiology in whom clinically relevant VT or VF is induced at EPS may be candidates for ICD therapy. In these patients, the induced arrhythmia is presumed to be the cause for syncope.22,23 Follow-up studies of these patients have established that their annual cardiovascular mortality rate averages 20%, with a large proportion of sudden, presumably arrhythmic, deaths.23 In this patient subset, ICD therapy is often applied with results comparable with those obtained in patients with sustained VT.12 ICD therapy currently is a class I indication for patients with syncope of unknown etiology and inducible VT or VF.1
Prophylaxis After Acute Myocardial Infarction
SCD is a devastating but increasingly infrequent complication after acute MI. Delayed VT or VF was originally linked to post-infarction ventricular arrhythmias, poor left ventricular function, impaired heart rate variability, and other risk factors.24 Two large clinical trials, Defibrillator in Acute Myocardial Infarction Trial (DINAMIT) and Immediate Risk Stratification Improves Survival (IRIS), have studied post-infarction populations with risk factors for potential benefits of ICD therapy.25,26 In DINAMIT, patients had reduced left ventricular function (mean, 28%) and impaired autonomic function (depressed heart rate variability or an elevated average 24-hour heart rate ≥80 beats/min on Holter monitoring), whereas in IRIS risk factors included reduced left ventricular function (mean, 35%) and a heart rate of 90 beats/min or higher or nonsustained VT at a heart rate of 150 beats/min or higher on Holter monitoring with any LVEF.26 Neither trial demonstrated the benefits of ICD therapy in the immediate post-infarction period, defined as 6 to 40 days in DINAMIT and less than 30 days (mean, 13 days) in IRIS.23,24
Nonischemic Dilated Cardiomyopathy
Nonischemic dilated cardiomyopathy (DCM) is associated with a high mortality rate within 2 years of diagnosis, with a minority of patients surviving 5 years. Approximately half of these deaths are sudden and unexpected.27 The combination of poor left ventricular function and frequent episodes of nonsustained VT in these patients is associated with an increased risk of SCD.28 Unlike in ischemic heart disease, the value of EPS is limited.29 The efficacy of drug therapy is low in the presence of impaired left ventricular systolic function and is difficult to predict on the basis of invasive or noninvasive testing. ICD implantation may be preferred in the management of symptomatic patients with this condition as well as VT or VF. ICD therapy can also be used as the bridge to orthotopic heart transplantation in many of these patients. The Cardiomyopathy Trial (CAT) was undertaken as a pilot study to evaluate the potential benefit of prophylactic ICD implantation on all-cause mortality in patients with DCM. The trial was terminated early because no appreciable benefit of ICD implantation was observed. However, the trial group was small, the event rate was extremely low, and the follow-up was abbreviated. In contrast, in the AVID study, these patients derived benefits similar to those derived by patients with CAD and had similar event rates. The optimal management of idiopathic DCM, especially the role of ICD in the primary prevention of SCD, has been defined by recently completed large trials (Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation [DEFINITE], SCD-HeFT). These trials enrolled patients with DCM and moderate left ventricular systolic dysfunction (ejection fraction <35%) with or without ventricular arrhythmias. Although DEFINITE showed no overall mortality benefit with the ICD, a large subgroup (NYHA class III patients) showed a favorable trend to survival improvement with the ICD (P = .06).31 The SCD-HeFT population showed survival benefit with the ICD.2 These data support the prophylactic use of ICD therapy in nonischemic DCM with moderately impaired systolic left ventricular function.
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a diverse group of disorders that have, as a common feature, primary hypertrophy of the left ventricle. Their prevalence has recently been estimated to be as high as 1 in 500, making this group of diseases the most common genetically transmitted cardiovascular diseases.32 SCD, which may be the first manifestation of the disease in an asymptomatic individual, has been reported annually in up to 4% of these patients.33 Malignant ventricular tachyarrhythmias have been described as a mechanism for SCD in adults with this condition.33,34 Risk factors for SCD include syncope, a very young age at presentation, extreme degrees of ventricular hypertrophy, a strong family history of SCD from cardiac causes, and nonsustained VT.32–33 Studies of patients resuscitated from cardiac arrest indicate that many patients will have another event. In contrast to other cardiomyopathies, EPS may be of prognostic significance because some studies have shown that inducible sustained ventricular arrhythmias appear to be associated with cardiac arrest and syncope.35 Pharmacologic therapy, in the form of β-blockers or calcium channel antagonists, has frequently been used. Although outflow tract gradients may diminish with medical therapy, it has, at best, marginal efficacy in preventing SCD. The empirical use of amiodarone has been recently reported to be associated with improved survival.34 However, risk stratification and the prediction of drug efficacy remains difficult and controversial. High-risk patients with HCM and SCD survivors should be considered for ICD therapy instead of, or in conjunction with, drug therapy.1
The limited efficacy of medical therapy combined with compliance issues associated with long-term drug administration in young patients makes device therapy an attractive option. Maron and associates published a multi-center retrospective study of the efficacy of ICD therapy in 128 HCM patients thought to be at high risk for SCD.36 Thirty-four percent of these patients had ICDs implanted for secondary prevention after cardiac arrest, whereas 66% had devices implanted for primary prevention because of the presence of risk factors listed earlier. During a mean follow-up of 3 years, appropriate therapy was delivered in 23% of patients. Two patients died suddenly despite ICD therapy. On a cautionary note, a significant rate of complications was associated with ICD therapy, with inappropriate therapy being delivered to 25% of patients. ICD therapy is indicated in patients with HCM for secondary prevention after sustained VT or VF.1 Primary prevention is considered a class IIb indication for ICD implantation. The results of ICD therapy in HCM should lead to greater use of device therapy in high-risk patients.36 Future directions of research include better characterization of the specific myosin gene mutations associated with shorter life expectancy, which may target candidates for primary prevention with early use of amiodarone, defibrillator therapy, or both.37
Heart Failure Populations
In the secondary prevention studies, the role of left ventricular dysfunction as an important determinant of the survival benefit of defibrillator therapy was highlighted.14,38,39 Survival of ICD recipients is strongly influenced by left ventricular systolic function. Patients with LVEF less than 30% have inferior survival rates at 3 years of follow-up compared with those with better left ventricular function. However, both populations appear to derive a significant survival benefit from ICD implantation. In an early analysis, we demonstrated that patients with LVEF less than 36% derived the benefit of defibrillator therapy over drug therapy.17 Similar data were noted in the CIDS and MADIT-I studies. The MADIT-II study evaluated the hypothesis that patients with CAD and MI who had an LVEF less than 30% would have improved survival with defibrillator therapy compared with conventional heart failure therapy.3 The trial demonstrated a 31% reduction in relative risk, which was estimated to decline from a projected 19% 2-year mortality rate to an actual 12% mortality rate with defibrillator insertion. Both appropriate and inappropriate shock deliveries have been noted during follow-up (see Figure 85-3). However, analyses have identified the subgroup deriving the most benefit. These appear to be patients with prolonged QRS duration and left ventricular dysfunction. However, a recent analysis suggests that patients in the ICD arm have similar rapid VT or VF events detected by the ICD, whether or not the QRS duration was above or below 140 ms.40 SCD-HeFT compared survival after ICD insertion or empiric amiodarone with placebo in patients with ischemic or nonischemic NYHA classes II and III heart failure and LVEF below 35%.2 The overall mortality rate in the placebo arm (7.2% annually) was not altered by amiodarone therapy but was greatly reduced by ICD insertion (hazard ratio [HR], 0.77; P = .007). This effect was more obvious in class II patients (HR, 0.54), with LVEF less than 31%, and prolonged QRS duration (HR, 0.67). The Comparison of Medical Therapy, Pacing and Defibrillator Trial (COMPANION) demonstrated improved survival in NYHA classes III and IV heart failure patients with a prolonged QRS, LVEF less than 35%, and a left ventricular diameter greater than 60 mm with a biventricular ICD compared with pacing or conventional heart failure drug therapy.41 A 43% reduction in mortality rate was observed with biventricular ICD insertion as prophylaxis for SCD. The recently completed MADIT CRT and RAFT trials, reviewed in detail in Chapter 83, provide strong evidence to support improved survival in patients with left ventricular systolic dysfunction, ventricular dyssynchrony demonstrated by wide QRS on electrocardiogram, and class I or II heart failure symptoms.41,42 The recently released update to the European Society of Cardiology (ESC) guidelines for cardiac resynchronization therapy now accepts the use of a cardiac resynchronization therapy (CRT) device, including ICD therapy, in patients with NYHA class II heart failure, QRS duration of 150 ms or greater, and left ventricular systolic dysfunction manifested as an EF of 35% or less.43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree