Early use of β blockers (BBs) in acute myocardial infarction remains controversial, with some studies demonstrating benefit and others harm. The aim of this study was to assess the association between pre–percutaneous coronary intervention (PCI) BB use and in-hospital outcomes in patients who underwent primary PCI for ST-segment elevation myocardial infarction between 2007 and 2009 at institutions participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC-2). Inverse propensity score weighting was used to account for the nonrandomized use of pre-PCI BBs. The cohort comprised 7,667 patients, with 4,769 (62%) receiving pre-PCI BBs. These patients were older, with higher rates of diabetes mellitus, hypertension, and previous myocardial infarction, PCI, or coronary artery bypass grafting. In adjusted models, pre-PCI BB use was associated with lower rates of intraprocedural ventricular tachycardia or ventricular fibrillation (odds ratio [OR] 0.58, p <0.01) and lower in-hospital mortality (OR 0.65, p = 0.022), with increases in rates of emergent coronary artery bypass grafting (OR 1.56, p <0.01) and repeat PCI (OR 1.93, p <0.01). There were no significant increases in rates of cardiogenic shock and congestive heart failure. In conclusion, pre-PCI BB use in this population was associated with decreased arrhythmia and mortality, without increasing rates of cardiogenic shock and heart failure but with higher rates of repeat PCI and emergent coronary artery bypass grafting, suggesting that there may yet remain a role for early BB use in pre-PCI patients with ST-segment elevation myocardial infarctions.
There is a paucity of data on the clinical impact of pre–percutaneous coronary intervention (PCI) β-blocker (BB) use in patients with ST-segment elevation myocardial infarctions (STEMIs) who undergo primary PCI, the preeminent therapy for STEMI in the United States. The purpose of this study was to evaluate the relation between pre-PCI BB use and in-hospital outcomes in a large cohort of patients with STEMIs in a large regional multicenter quality improvement collaborative registry: the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC-2).
Methods
The study population was composed of all patients who underwent primary PCI for STEMI at 32 participating hospitals across Michigan from January 2007 through December 2009. The BMC-2 registry has been described previously. Patients were included if they underwent PCI <12 hours after symptom onset. Patients were excluded if they underwent rescue PCI or presented after cardiac arrest. Demographics, baseline clinical characteristics, and home medications of the included patients were collected in addition to their periprocedural and in-hospital outcomes.
Patients were divided into 2 groups: those receiving BBs before they underwent PCI (pre-PCI BB use) and those not receiving BBs. Pre-PCI BB administration was defined as receipt of a BB <24 hours before PCI. Pre-PCI BB use included taking BBs as part of usual home medicines, administration during initial triage, or during catheterization but before PCI.
Differences in baseline characteristics between groups were evaluated using chi-square tests for categorical variables and Student’s t tests for continuous variables. We carried out unadjusted and adjusted analysis to estimate the odds ratios (ORs) for intra- and periprocedural morbidity and mortality. In the unadjusted analysis, generalized linear models for repeated measures with a logit link were used. Models for repeated measures were used to account for the clustering of patients within hospitals.
Adjusted analysis using inverse propensity score weighting was used to account for potential confounders due to the nonrandomization of the comparison groups. Specifically, we modeled the probability of receiving the treatment (pre-PCI BB) conditional on all available preprocedural variables (i.e., the propensity score) using a logistic regression model. The estimated propensity scores were then used to weight the contribution of each subject in the generalized linear models as the inverse of the propensity score (or 1 − propensity score) for subjects in the treatment group (or the control group). The variables adjusted for included age, gender, race, smoking status, angiographic characteristics, history of hypertension, congestive heart failure, diabetes mellitus, end-stage renal disease requiring hemodialysis, significant valvular heart disease, current or recent gastrointestinal bleeding, chronic obstructive pulmonary disease, previous myocardial infarction, PCI, coronary artery bypass grafting (CABG), and body mass index. We used the Cochran-Mantel-Haenszel test for categorical variables and analysis of variance for continuous variables to evaluate differences in adjustment variables by treatment versus nontreatment within strata formed by quintiles of propensity scores to ensure balance achieved by inverse propensity scoring.
We also identified high-risk patient subgroups for further analysis, defined by gender, age ≤59 or ≥60 years, history of diabetes, and culprit lesion location (left anterior descending, right, and left circumflex coronary arteries). We evaluated each subgroup for differences in the development of cardiogenic shock, intraprocedural ventricular arrhythmia requiring therapy (intraprocedural ventricular tachycardia [VT] or ventricular fibrillation [VF]), and in-hospital mortality.
Results
We identified 7,667 patients who underwent primary PCI at BMC-2-affiliated institutions from January 2007 to December 2009. Of these, 2,898 patients (38%) did not receive pre-PCI BBs, compared with 4,769 (62%) who received pre-PCI BBs. Baseline characteristics are listed in Table 1 . Most patients were men (71%). Patients who received pre-PCI BBs were more likely to have histories of hypertension, diabetes, or heart failure. They tended to be slightly older and were more likely to have had previous myocardial infarctions, PCI, or CABG. Patients receiving BBs were more likely to present with heart failure and to have higher presenting serum creatinine levels. There were no statistically significant differences in gender or race between those receiving pre-PCI BBs and those not.
| Variable | No Preprocedural BB Use (n = 2,898) | Preprocedural BB Use (n = 4,769) | p Value |
|---|---|---|---|
| Women | 817 (28.2%) | 1,378 (28.9%) | 0.509 |
| Race | 0.8925 | ||
| White | 2,447 (84.4%) | 4,034 (84.6%) | |
| Black | 313 (10.8%) | 519 (10.9%) | |
| Other | 138 (4.8%) | 216 (4.5%) | |
| Current smokers | 1,400 (48.3%) | 2,140 (44.9%) | 0.0034 |
| Hypertension | 1,807 (62.4%) | 3,643 (76.4%) | <0.0001 |
| Heart failure | 130 (4.5%) | 426 (8.9%) | <0.0001 |
| Renal insufficiency requiring hemodialysis | 17.0 (0.6%) | 56.0 (1.2%) | 0.0102 |
| Significant valvular disease | 46.0 (1.6%) | 111 (2.3%) | 0.0265 |
| Current or recent gastrointestinal bleed | 26.0 (0.9%) | 76.0 (1.6%) | 0.0099 |
| Chronic obstructive pulmonary disease | 369 (12.7%) | 711 (14.9%) | 0.0079 |
| Atrial fibrillation | 146 (5.0%) | 349 (7.3%) | <0.0001 |
| Previous cardiac arrest | 43.0 (1.5%) | 76.0 (1.6%) | 0.706 |
| Previous myocardial infarction | 441 (15.2%) | 1,334 (28.0%) | <0.0001 |
| Previous PCI | 495 (17.1%) | 1,501 (31.5%) | <0.0001 |
| Previous CABG | 118 (4.1%) | 415 (8.7%) | <0.0001 |
| Diabetes mellitus | 578 (19.9%) | 1,290 (27.0%) | <0.0001 |
| Extravascular atherosclerotic disease | 328 (11.3%) | 841 (17.6%) | <0.0001 |
| Body mass index (kg/m 2 ) | <0.0001 | ||
| Mean ± SD | 29.14 ± 6.32 | 29.83 ± 6.92 | |
| Median (IQR) | 28.19 (25.09–32.12) | 28.84 (25.68–32.94) | |
| Age (yrs) | 0.0018 | ||
| Mean ± SD | 59.44 ± 13.07 | 60.39 ± 12.88 | |
| Median (IQR) | 58.00 (50.00–68.00) | 59.00 (51.00–69.00) | |
| Creatinine on presentation (mg/dl) | 0.0099 | ||
| Mean ± SD | 1.11 ± 0.62 | 1.16 ± 0.87 | |
| Median (IQR) | 1.00 (0.90–1.20) | 1.00 (0.90–1.20) | |
| Hemoglobin on presentation (g/dl) | 0.1297 | ||
| Mean ± SD | 14.36 ± 2.02 | 14.28 ± 2.08 | |
| Median (IQR) | 14.50 (13.30–15.60) | 14.50 (13.10–15.70) | |
| Ostial culprit atherosclerotic lesion | 184 (6.3%) | 325 (6.8%) | 0.4271 |
| Calcium in culprit atherosclerotic lesions | 213 (7.3%) | 297 (6.2%) | 0.0559 |
| Chronic total occlusion | 22.0 (0.8%) | 46.0 (1.0%) | 0.3523 |
| Restenotic culprit atherosclerotic lesion | 85.0 (2.9%) | 344 (7.2%) | <0.0001 |
| Bifurcating culprit atherosclerotic lesion | 132 (4.6%) | 234 (4.9%) | 0.4836 |
In our unadjusted analysis ( Table 2 ), pre-PCI BB use was associated with lower procedural mortality (OR 0.50, 95% confidence interval [CI] 0.27 to 0.94), intraprocedural cardiogenic shock (OR 0.75, 95% CI 0.58 to 0.96), and intraprocedural VT or VF (OR 0.60, 95% CI 0.46 to 0.78). However, patients receiving pre-PCI BBs were more likely to have subacute stent thrombosis (OR 1.83, 95% CI 1.17 to 2.87) and to undergo emergent CABG (OR 1.58, 95% CI 1.09 to 2.28) or repeat PCI (OR 1.68, 95% CI 1.12 to 2.52). They were also more likely to develop congestive heart failure during their hospitalizations (OR 1.24, 95% CI 1.01 to 1.52) or to develop renal failure requiring hemodialysis (OR 2.19, 95% CI 1.42 to 3.37). There was a trend toward decreased all-cause in-hospital mortality in those patients receiving pre-PCI BBs, but this did not reach statistical significance.
| Outcome | Unadjusted Analysis | Propensity Score–Adjusted Analysis | ||
|---|---|---|---|---|
| OR (95% CI), BB vs No BB | p Value | OR (95% CI), BB vs No BB | p Value | |
| Intraprocedural VT or VF requiring therapy | 0.60 (0.46–0.78) | 0.0001 | 0.58 (0.44–0.76) | <0.0001 |
| Intraprocedural cardiogenic shock | 0.75 (0.58–0.96) | 0.0217 | 0.67 (0.52–0.86) | 0.0016 |
| Intraprocedural mortality | 0.50 (0.27–0.94) | 0.0305 | 0.41 (0.22–0.76) | 0.0049 |
| Subacute stent thrombosis | 1.83 (1.17–2.87) | 0.0083 | 1.80 (1.09–2.99) | 0.0227 |
| Repeat PCI | 1.68 (1.12–2.52) | 0.0122 | 1.93 (1.30–2.87) | 0.0011 |
| Emergent CABG | 1.58 (1.09–2.28) | 0.0155 | 1.56 (1.12–2.16) | 0.0088 |
| VT or VF requiring therapy | 0.96 (0.75–1.23) | 0.7542 | 0.94 (0.74–1.20) | 0.6204 |
| Atrial fibrillation requiring therapy | 1.34 (1.05–1.71) | 0.0202 | 1.18 (0.94–1.48) | 0.1597 |
| Stroke or transient ischemic attack | 0.90 (0.50–1.61) | 0.7136 | 0.67 (0.33–1.37) | 0.2708 |
| Congestive heart failure | 1.24 (1.01–1.52) | 0.0364 | 0.96 (0.77–1.20) | 0.7163 |
| Nephropathy requiring hemodialysis | 2.19 (1.42–3.37) | 0.0004 | 1.21 (0.68–2.14) | 0.5218 |
| Cardiogenic shock | 1.01 (0.72–1.43) | 0.9525 | 0.83 (0.58–1.19) | 0.3152 |
| All-cause in-hospital mortality | 0.82 (0.59–1.12) | 0.2138 | 0.65 (0.45–0.94) | 0.0218 |
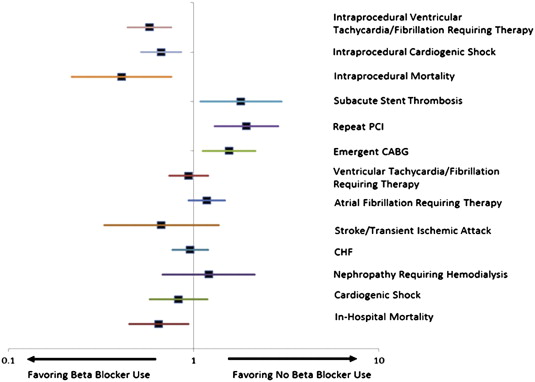
Results of the propensity score adjusted analysis are listed in Table 2 and shown in Figure 1 . The adjustment variables did not differ significantly between those receiving pre-PCI BBs and those not within each quintile of propensity scores, indicating balance in the propensity score model ( Appendix ). Pre-PCI BB use was associated with less intraprocedural VT or VF (OR 0.58, 95% CI 0.44 to 0.76) or cardiogenic shock (OR 0.67, 95% CI 0.52 to 0.86) and less intraprocedural mortality (OR 0.41, 95% CI 0.22 to 0.76). Increased associations of pre-PCI BB use with subacute stent thrombosis (OR 1.80, 95% CI 1.09 to 2.99), repeat PCI (OR 1.93, 95% CI 1.30 to 2.87), and emergent CABG (OR 1.56, 95% CI 1.12 to 2.16) remained despite adjustment. There was no significant association between BB use and the development of congestive heart failure or renal failure requiring hemodialysis in the adjusted analysis. Pre-PCI BB use was associated with a significant reduction in all-cause in-hospital mortality (OR 0.65, 95% CI 0.45 to 0.94).