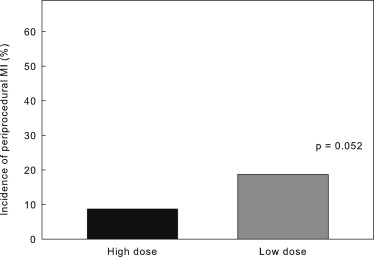
The aim of the present study was to investigate whether percutaneous coronary intervention–related periprocedural myocardial infarction (MI) can be suppressed more significantly with high- compared with low-dose rosuvastatin. A total of 232 patients scheduled to undergo elective percutaneous coronary intervention within 5 to 7 days were assigned to groups that would receive either 2.5 or 20 mg/day of rosuvastatin (n = 116 each). The incidence of periprocedural MI did not significantly differ between the high and low-dose groups (8.7% vs 18.7%, p = 0.052). In patients who were not taking statins at the time of enrollment, high-dose rosuvastatin significantly suppressed periprocedural MI compared with the low dose (10.5% vs 30.0%, p = 0.037). The difference was not significant in patients who were already taking statins (high vs low dose 7.6% vs 10.6%, p = 0.582). In conclusion, the incidence of percutaneous coronary intervention–related periprocedural MI was reduced more effectively by high-dose than by low-dose rosuvastatin in statin-naive patients. However, low-dose rosuvastatin is sufficient for patients who are already taking statins.
The anti-ischemic effect of statins against percutaneous coronary intervention (PCI)–related procedural myocardial infarction (MI) has been determined mostly in statin-naive patients or in patients with acute coronary syndromes. Whether periprocedural statins can also protect patients with stable coronary artery disease (CAD) who take statins before PCI remains unknown. Therefore, the aim of this prospective study was to examine whether a high-dose statin administered before elective PCI for stable CAD can reduce periprocedural myocardial injury in patients who are statin naive and in those who have already received statins.
Methods
This prospective, multicenter, randomized, parallel-group, open-label study took place at 7 institutions. The aim was to investigate whether PCI-related periprocedural complications can be suppressed more significantly with high-dose compared with low-dose rosuvastatin. The inclusion criteria were typical stable effort angina, positive stress test findings (electrocardiography, nuclear imaging, or stress echocardiography), and candidacy for elective PCI. Patients were excluded if they had unstable angina requiring emergency PCI, increases in creatine kinase-MB (CK-MB) higher than the upper normal limit at the time of randomization, liver damage with liver enzymes (aspartate aminotransferase or alanine aminotransferase) >100 IU/dl or total bilirubin >3.0 mg/dl, renal failure with creatinine >2.0 mg/dl, or histories of adverse effects caused by any type of statins. Patients who were undergoing current therapy with statins were included, except those who received statin doses higher than the standard dose. When a patient had been taking statins for <2 weeks, enrollment was postponed until the duration of statin therapy exceeded 2 weeks. From September 1, 2007, to March 31, 2011, 232 patients (mean age 68 ± 10 years, 152 men) were enrolled in the study, of whom 116 each were randomized to receive low (2.5 mg/day) and high (20 mg/day) doses of rosuvastatin starting from 5 to 7 days before the planned intervention.
According to our standard protocol, all patients without contraindications were administered aspirin (100 mg/day) and ticlopidine 100 mg twice daily ≥7 days before or clopidogrel (75 mg/day) ≥4 days before or as a loading dose of 300 mg ≥6 hours before the procedure. All patients continued with ticlopidine (100 mg twice daily) or clopidogrel (75 mg/day) for 1 month, and those with drug-eluting stents continued these drugs for 6 months. Before intervention, patients received weight-adjusted intravenous heparin with a target activated clotting time of >250 seconds. Coronary stents were implanted with or without predilation. The sizes of stents were determined by intravascular ultrasonography performed using motorized pullback at a constant speed of 0.5 mm/second during PCI or quantitative coronary angiography. Procedural success was defined as a reduction of stenosis to <30% residual narrowing. Data from patients who did not undergo PCI because of the absence of suitable lesions (including no stenosis), those who underwent PCI for left main trunk lesions or chronic totally occluded lesions, and those who underwent PCI with rotablators or distal protection devices were excluded from the analysis.
Blood samples were collected before and at 6, 12, and 24 hours after the procedure. CK-MB and high-sensitivity troponin T (HSTnT) were measured using an electrochemiluminescence immunoassay (SRL, Tokyo, Japan). The upper normal limits were defined as 5 and 0.014 ng/ml for CK-MB and HSTnT, respectively. Clinical follow-up data were collected 1 year later either from medical charts or by telephone interviews. The ethics committees at the participating institutions approved all study protocols, and each patient provided written informed consent to participate. The study was not supported by any external source of funding.
The primary end point was in-hospital death or the occurrence of MI, defined as a postprocedural increase in CK-MB 3 times the upper normal limit. Secondary end points included myocardial injury, defined as a postprocedural increase in HSTnT of >0.1 ng/ml; the absence of coronary reflow, defined as Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 or 1 ; slow flow, defined as TIMI flow grade 2 ; and all major adverse cardiac events (cardiac death, MI, and need for unplanned revascularization) that occurred <1 year after PCI.
The sample size was determined on the basis of previous studies to demonstrate a reduction in the primary end point from 18% in the low-dose group and 5% in the high-dose group (2-sided chi-square test, α = 0.05, power = 0.83). We also compared data from patients who were statin naive and from those who were already receiving statins. Statin-naive patients in the high- and low-dose groups were defined as groups A and C, respectively, and patients receiving statin therapy in the high- and low-dose groups were defined as groups B and D, respectively. Continuous variables between the 2 groups were compared using Student’s t tests for normally distributed values (age, the left ventricular ejection fraction) and Mann-Whitney U tests. Continuous variables among 4 groups (groups A, B, C, and D) were compared using 1-way analyses of variance or Wilcoxon’s tests. Proportions were compared using chi-square tests or Fisher’s exact tests as appropriate. Odds ratios and 95% confidence intervals indicating the risk for periprocedural myocardial injury associated with different factors were assessed using multiple logistic regression adjusted for age; gender; use of β blockers, renin-angiotensin system inhibitors, nitrates, or nicorandil; diabetes; dyslipidemia; systemic hypertension; type of lesion (A/B1 vs B2/C) ; multivessel intervention; stent length; direct stenting; and high pressure after dilatation. Two-tailed p values <0.05 were considered significant. Data were analyzed using SigmaPlot version 11 (Systat Software, Inc., San Jose, California). Results are expressed as mean ± SD unless otherwise specified.
Results
Among the 232 patients enrolled in the study, 10 and 12 patients from the low- and high-dose groups, respectively, were excluded because of stenoses not being found (n = 4 in each group), contraindications to PCI (n = 4 and n = 5 in the low- and high-dose groups, respectively), use of a rotablator (n = 1 in the high-dose group), misadministration of assigned dose (n = 1 in the low-dose group), and sampling error (n = 2 and n = 1 in the low- and high-dose groups, respectively). Therefore, we analyzed data from 106 and 104 patients in the low- and high-dose groups, respectively. Table 1 lists the clinical background of the patients. Table 2 lists the clinical background of the statin-naive group A and C patients (high and low dose, respectively), and statin-treated group B and D patients (high and low dose, respectively). Table 3 lists changes in low-density lipoprotein and high-density lipoprotein cholesterol levels.
| Variable | Rosuvastatin Dose (mg) | p Value | |
|---|---|---|---|
| 20 (n = 104) | 2.5 (n = 106) | ||
| Age (yrs) | 69 ± 10 | 68 ± 9 | 0.993 |
| Men | 81 (77.9%) | 81 (76.4%) | 0.929 |
| Symptoms | |||
| Asymptomatic | 23 (22.1%) | 26 (24.5%) | 0.742 |
| Stable angina pectoris | 78 (75%) | 78 (73.5%) | |
| Unstable angina pectoris | 3 (2.9%) | 2 (1.9%) | |
| Diabetes mellitus | 54 (50.0%) | 55 (51.9%) | 0.892 |
| Low-density lipoprotein cholesterol (mg/dl) | 112.4 ± 25.8 | 113.0 ± 28.1 | 0.873 |
| High-density lipoprotein cholesterol (mg/dl) | 46.7 ± 9.5 | 47.4 ± 11.8 | 0.828 |
| Triglyceride (mg/dl) | 138.2 ± 59.5 | 135.6 ± 66.8 | 0.636 |
| Hypertension | 80 (76.9%) | 76 (71.7%) | 0.479 |
| Current smoker | 26 (25.0%) | 31 (29.2%) | 0.592 |
| Previous MI | 41 (39.4%) | 44 (41.5%) | 0.867 |
| Chronic kidney disease (stage ≥III) | 18 (17.3%) | 16 (15.1%) | 0.804 |
| Left ventricular ejection fraction (%) | 58.3 ± 13.7 | 58.5 ± 13.2 | 0.938 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 76 (73.1%) | 79 (74.5%) | 0.934 |
| β blockers | 63 (60.65) | 61 (57.55) | 0.760 |
| Calcium channel blockers | 44 (38.5%) | 33 (31.1%) | 0.332 |
| Statins | 66 (63.5%) | 65 (61.3%) | 0.859 |
| Variable | Rosuvastatin Dose (mg) | p Value | |||
|---|---|---|---|---|---|
| 20 | 2.5 | ||||
| Group A (n = 38) | Group B (n = 66) | Group C (n = 40) | Group D (n = 66) | ||
| Age (yrs) | 68 ± 10 | 69 ± 11 | 68 ± 8 | 69 ± 9 | 0.914 |
| Men | 31 (81.6%) | 50 (75.8%) | 34 (85%) | 47 (71.2%) | 0.360 |
| Diabetes mellitus | 19 (50.0%) | 33 (50.0%) | 25 (62.5%) | 30 (45.4%) | 0.396 |
| Hypertension | 28 (73.7%) | 52 (78.8%) | 30 (75%) | 46 (69.7%) | 0.695 |
| Current smokers | 13 (36.1%) | 13 (19.7%) | 11 (27.5%) | 20 (30.3%) | 0.305 |
| Previous MI | 12 (31.6%) | 29 (43.9%) | 13 (32.5%) | 31 (46.9%) | 0.285 |
| Chronic kidney disease (stage ≥III) | 6 (15.8%) | 12 (18.2%) | 6 (15%) | 10 (15.2%) | 0.962 |
| Left ventricular ejection fraction (%) | 57.6 ± 12.4 | 58.8 ± 14.5 | 59.4 ± 14.7 | 57.9 ± 12.5 | 0.922 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 30 (78.9%) | 46 (69.7%) | 31 (82.5%) | 48 (72.7%) | 0.701 |
| β blockers | 24 (63.1%) | 38 (57.6%) | 25 (62.5%) | 36 (54.5%) | 0.789 |
| Calcium channel blockers | 14 (36.8%) | 30 (45.4%) | 18 (45%) | 25 (37.8%) | 0.724 |
| Powerful statins † | — | 52 (78.8%) | — | 50 (75.8%) | 0.835 ∗ |
| Variable | Group A (n = 38) | Group B (n = 66) | Group C (n = 40) | Group (n = 66) |
|---|---|---|---|---|
| Low-density lipoprotein cholesterol (mg/dl) | ||||
| Day of enrollment | 132.1 ± 17.8 | 100.8 ± 22.6 ∗ | 136.6 ± 18.8 | 98.0 ± 22.1 † |
| Day of PCI | 78.1 ± 11.1 †,‡ | 81.2 ± 15.5 ‡,§ | 90.5 ± 15.7 ‡ | 89.3 ± 18.0 ‡ |
| High-density lipoprotein cholesterol (mg/dl) | ||||
| Day of enrollment | 46.2 ± 7.4 | 47.0 ± 10.5 | 46.2 ± 10.2 | 48.1 ± 12.7 |
| Day of PCI | 41.5 ± 6.8 ‡ | 45.7 ± 10.1 | 43.4 ± 9.6 ‡ | 47.4 ± 13.2 |
| Triglyceride (mg/dl) | ||||
| Day of enrollment | 137.2 ± 58.4 | 139.4 ± 60.2 | 132.6 ± 70.1 | 138.4 ± 66.6 |
| Day of PCI | 139.1 ± 61.0 | 136.6 ± 58.4 | 136.1 ± 66.4 | 134.4 ± 62.8 |
‡ p <0.01 versus day of enrollment.
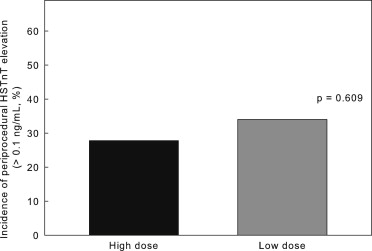
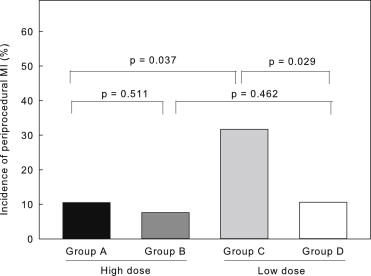
Angiographic features and PCI contents did not significantly differ between the high- and low-dose groups or among groups A, B, C, and D. None of the patients died in the hospital. Periprocedural MI, defined as CK-MB elevation, developed in 29 patients (13.8%) <24 hours after PCI. The incidence of periprocedural MI did not significantly differ between the high- and low-dose groups (8.7% vs 18.7%, p = 0.052; Figure 1 ). Periprocedural myocardial injury, defined as HSTnT elevation, occurred in 66 patients (31.4%), including all 29 with CK-MB elevations. The incidence of periprocedural injury also did not significantly differ between the high- and low-dose groups (27.8% vs 34.0%, p = 0.609; Figure 2 ). However, the incidence of periprocedural MI was significantly lower in the statin-naive patients in the high-dose (group A) than in the low-dose (group C) group ( Figure 3 ), indicating that more protection was conferred by high-dose than by low-dose rosuvastatin. Similarly, the incidence of periprocedural myocardial injury determined by HSTnT elevation was significantly lower in group A than in group C ( Figure 4 ). Figures 3 and 4 show that in patients who were treated with statins before they underwent procedures, the incidence of periprocedural MI or myocardial injury did not significantly differ between group B (high dose) and group D (low dose), indicating that high-dose rosuvastatin did not potentiate the protection conferred by current statin therapy. The incidence of CK-MB or HSTnT elevation significantly differed between groups C and D ( Figures 3 and 4 ) but not between groups A and B ( Figures 3 and 4 ), indicating that the disadvantage of statin-naive status could be overcome by high- but not by low-dose rosuvastatin.