Chapter 40 Imaging of Myocardial Metabolism
METHODS TO IMAGE MYOCARDIAL METABOLISM
Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy offers the advantages of the ability to measure multiple metabolic pathways simultaneously, the relative ease in performing serial measurements, and the lack of ionizing radiation. Moreover, when combined with MRI, near-simultaneous measurements of myocardial perfusion and mechanical function are possible. A number of biologically important nuclei can be measured, including phosphorous (31P), hydrogen (1H), carbon (13C), sodium (23Na), nitrogen (15N), and fluorine (19F). The fundamental principle of MRS is that the chemical environment of nuclei induces local magnetic fields that shift their resonance frequency. The different frequency shift for different metabolites results in a signal consisting of one or more discrete resonance frequencies. The Fourier transform of the acquired signal produces a spectrum with peaks at distinct frequencies. The MRS spectrum displays the signal intensity as a function of frequency measured in parts per million, relative to the frequency of a reference compound. The signal intensity at a given frequency is proportional to the amount of the respective metabolite and can be used to determine the absolute concentration of the metabolite using appropriate calibrating reference signal.1,2
MRS is limited by low signal-to-noise, concomitant limited spatial resolution, intravoxel signal contamination, and long acquisition times. Compared with nuclear imaging methods, MRS has a much lower sensitivity (detecting millimolar as opposed to nanomolar concentrations). As a consequence, the initial success of imaging of cardiac metabolism using Carbon-13 labeled agents in intact animals has not been translated to the study of humans.3 Of note, cardiac applications for MRS become more limited as one moves from rodent to man as opposed to nuclear methods where the reverse occurs. This appears to be a function of both the higher field strength in the small-bore systems and the use of radiofrequency coils that are in closer proximity to the entire heart used in small-animal imaging. In contrast to rodent hearts, where measurements of the entire left ventricular (LV) myocardium are obtained, measurements in human myocardium are typically limited to the anterior myocardium. Currently, only 31P and 1H have been widely used for in vivo clinical cardiac examinations focusing on myocardial energetics (31P) and lipid accumulation (1H).1,3,4
Single-Photon Emission Computed Tomography (SPECT) (See Chapter 2)
Metabolic processes that can be measured by SPECT include:
Positron Emission Tomography (PET) (See Chapter 3)
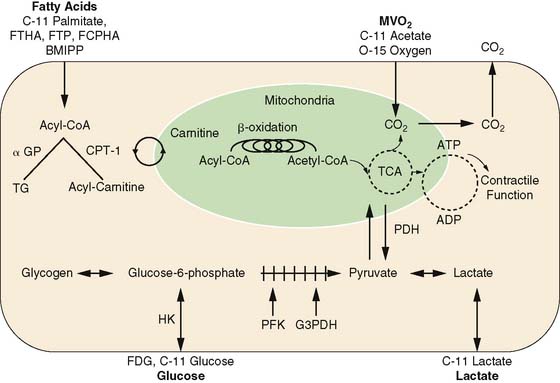
The major advantages of PET are its intrinsic quantitative capability and the use of radiopharmaceuticals labeled with the positron-emitting radionuclides. The PET detection scheme permits accurate quantification of activity in the field of view. The positron-emitting radionuclides of the biologically ubiquitous elements oxygen (15O), carbon (11C), and nitrogen (13N), as well as fluorine (18F) substituting for hydrogen, can be incorporated into a wide variety of substrates or substrate analogs that participate in diverse biochemical pathways without altering the biochemical properties of the substrate of interest (see Fig. 40-1). By combining the knowledge of the metabolic pathways of interest with kinetic models that faithfully describe the fate of the tracer in tissue, an accurate interpretation of the tracer kinetics as they relate to the metabolic process of interest can be achieved. The major disadvantages of PET are its complexity in both radiotracer design and image quantification schemes and expense. Metabolic processes that are typically measured with PET are myocardial oxygen consumption (MVO2), carbohydrate metabolism, and fatty acid metabolism.
Myocardial Oxygen Consumption (MVO2) (See Chapter 38)
Because oxygen is the final electron acceptor in all pathways of aerobic myocardial metabolism, PET with 15O-oxygen has also been used to measure MVO2. The approach provides a measure of myocardial oxygen extraction and measures MVO2 directly. Due to its short physical half-life, 15O-oxygen is readily applicable in studies requiring repetitive assessments, such as those with an acute pharmacologic intervention. Its major disadvantages are the need for a multiple-tracer study (to account for myocardial blood flow and blood volume) and fairly complex compartmental modeling to obtain the measurements.12–14
PET using 11C-acetate is the preferred method of measuring MVO2 noninvasively. After initial extraction, acetate, a two-carbon-chain free fatty acid, is rapidly converted to acetyl-CoA. The primary metabolic fate of acetyl-CoA is metabolism through the tricarboxylic acid (TCA) cycle. Because of the tight coupling of the TCA cycle and oxidative phosphorylation, the myocardial turnover of 11C-acetate reflects overall flux in the TCA cycle and thus overall oxidative metabolism, or MVO2. Either exponential curve fitting or compartmental modeling is used to calculate MVO2. The latter is typically preferable in situations of low cardiac output where marked splaying of the input function and spillover of activity from the lungs to the myocardium can decrease the accuracy of the curve-fitting method.15–19
Carbohydrate Metabolism (See Chapter 38)
Most studies of myocardial glucose metabolism with PET have used FDG. This radiotracer competes with glucose for facilitated transport into the sarcolemma, then for hexokinase-mediated phosphorylation. The resultant FDG-6-phosphate is trapped in the cytosol, and the myocardial uptake of FDG is thought to reflect overall anaerobic and aerobic myocardial glycolytic flux.20–23 The myocardial kinetics of FDG have been well characterized, the acquisition scheme is relatively straightforward, and its production has become routine, owing in part to the rapid growth of its clinical use in oncology. As such, it remains the most widely used tracer for determination of myocardial glucose metabolism. Regional myocardial glucose utilization can be assessed in either relative or absolute terms (i.e., in nmol/g−1/min−1). For quantification, a mathematical correction for the kinetic differences between FDG and glucose called the lumped constant must be used to calculate rates of glucose metabolism. Of note, this value may vary depending upon the prevailing plasma substrate and hormonal conditions, decreasing the accuracy of the measurement.22,24–26 Other disadvantages of FDG include the limited metabolic fate of FDG in tissue, precluding determination of the metabolic fate (i.e., glycogen formation versus glycolysis) of the extracted tracer and glucose, and limitations on the performance of serial measurements of myocardial glucose utilization because of the relatively long physical half-life of 18F.
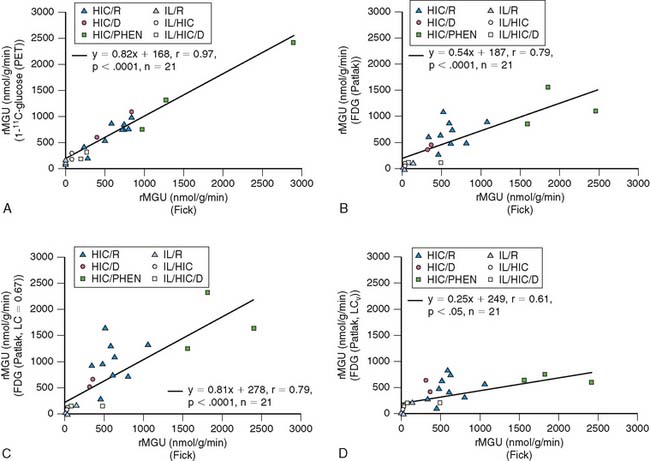
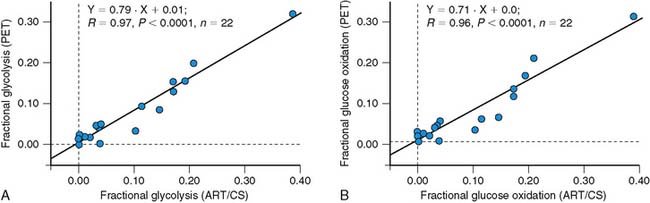
More recently, quantification of myocardial glucose utilization has been performed with PET using glucose radiolabeled in the 1-carbon position with carbon-11 glucose (11C-glucose). Because 11C-glucose is chemically identical to unlabeled glucose, it has the same metabolic fate as glucose, thus obviating the need for the lumped constant correction. It has been demonstrated that measurements of myocardial glucose utilization based on compartmental modeling of tracer kinetics are more accurate with 11C-glucose than with FDG, and can provide estimates of glycogen synthesis, glycolysis, and glucose oxidation (Figs. 40-2 and 40-3).27–29 Disadvantages of this method include compartmental modeling that is more demanding with 11C-glucose than it is with FDG, the need to correct the arterial input function for the production of 11CO2 and 11C-lactate, a fairly complex synthesis of the tracer, and the short physical half-life of 11C, requiring an on-site cyclotron.
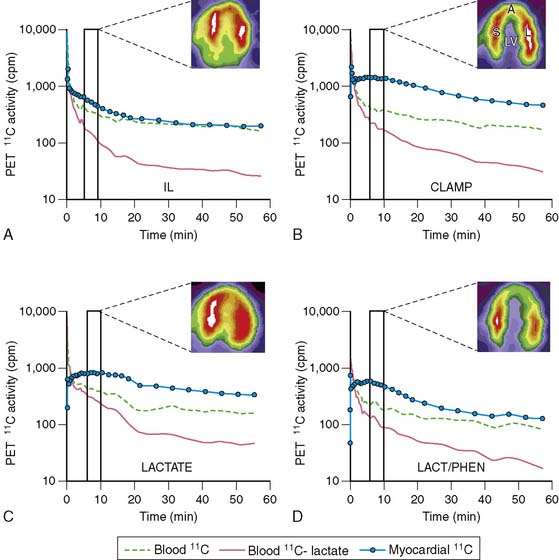
Lactate metabolism in the heart is a key source of energy production, particularly during periods of increased cardiac work. However, to date, the ability to measure myocardial lactate has been limited by the lack of availability of an appropriate radiotracer and analysis scheme. Recently, a multicompartment model was developed for the assessment of myocardial lactate metabolism using PET and L-3-11C-lactate. PET-derived extraction of lactate correlated well with lactate oxidation measured by arterial and coronary sinus sampling over a wide range of conditions (Fig. 40-4).30 This approach may help delineate the clinical role of lactate metabolism in a variety of pathologic conditions such as diabetes mellitus and myocardial ischemia. Moreover, when combined with either FDG or 11C-glucose, it permits a more comprehensive measurement of myocardial carbohydrate metabolism.
Fatty Acid Metabolism
The major advantage of 11C-palmitate is that its myocardial kinetics are identical to labeled palmitate. With appropriate mathematical modeling techniques, its use permits the assessment of various aspects of myocardial fatty acid metabolism such as uptake, oxidation, and storage.31–34 This level of metabolic detail is important because exactly which component of myocardial fatty acid metabolism is the main contributor to a pathologic process is frequently unclear. However, this method does suffer from several disadvantages, including reduced image quality and specificity, a more complex analysis, and the need for an on-site cyclotron and radiopharmaceutical production capability.
Most of the PET tracers in the category of fatty acid tracers that are trapped have been designed to reflect myocardial β-oxidation. One of the first radiotracers developed using this approach was 14-(R,S)-18F-fluoro-6-thiaheptadecanoic acid (FTHA). Initial results were promising, with uptake and retention in the myocardium according with changes in substrate delivery, blood flow, and workload in animal models.35,36 Moreover, PET with FTHA was used to evaluate the effects of various diseases such as coronary artery disease and cardiomyopathy on myocardial fatty acid metabolism.37,38 However, uptake and retention of FTHA has been shown to be insensitive to the inhibition of β-oxidation by hypoxia, reducing enthusiasm for this radiotracer to measure myocardial fatty acid metabolism.39 To circumvent this problem, 16-18F-fluoro-4-thiapalmitate (FTP) has been developed. This modification retains the metabolic trapping function of the radiotracer, which is proportional to fatty acid oxidation under normal oxygenation and hypoxic conditions.39,40 Similar to FDG, quantification of myocardial fatty acid metabolism with FTP requires the use of a lumped constant to correct for kinetic differences between the radiotracer and unlabeled palmitate. However, the stability of the lumped constant under most conditions is unknown. Thus, the ability to quantify fatty acid oxidation with this radiotracer is still unclear. Currently, FTP is undergoing commercialization and entering early phase-1 evaluation. Recently, a new 18F-labeled fatty acid radiotracer, trans-9(RS)-18F-fluoro-3,4(RS,RS) methylene heptadecanoic acid (FCPHA), has been developed.41 This radiotracer is also trapped after undergoing several steps of β-oxidation. Results of initial studies show that uptake of FCPHA into rat myocardium was approximately 1.5% of the injected dose per gram of tissue at 5 minutes, with little change over a period of 60 minutes, and low blood activity over the same period. However, the impact of alterations in plasma substrates, workload, and blood flow on myocardial kinetics is unknown. This radiotracer is also undergoing commercialization.
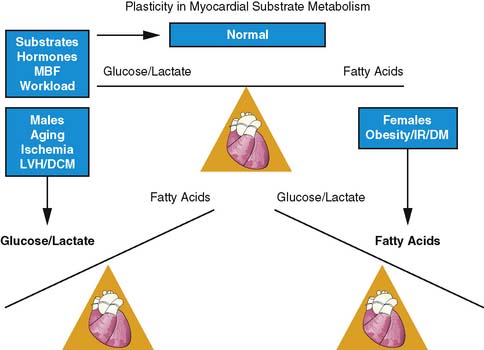
OVERVIEW OF MYOCARDIAL METABOLISM: NEED FOR FLEXIBILITY IN SUBSTRATE USE
The heart is an omnivore capable of switching between one substrate to another for energy production. This flexibility in substrate permits efficient response to various stimuli such as the pattern of substrate availability, the hormonal milieu, the level of tissue perfusion, and the amount of workload by the heart (Fig. 40-5).42,43 Control of substrate switching can represent either an acute or a chronic adaptation in response to either short or prolonged alterations in the physiologic environment. Inhibitory effects of fatty acid oxidation on glucose oxidation and increasing oxidation of glycogen, lactate, and glucose in response to increasing workload are examples of acute or short-term adaptations. Regulating these rapid changes are a host of enzymes such as pyruvate dehydrogenase complex and enzyme carnitine palmitoyl transferase-1, which is regulated by the concentration of malonyl-CoA.44–48
In contrast, chronic metabolic adaptations reflect alterations in gene expression regulating various metabolic pathways. These changes occur primarily at the transcriptional level through the coordinated up-regulation of enzymes and proteins in key metabolic pathways. A prominent example in this case is the nuclear receptor, peroxisome proliferator–activated receptor alpha (PPARα), which is a key regulator of myocardial fatty acid uptake, oxidation, and storage.49 For example, in diabetes mellitus, PPARα activity is increased, leading to an up-regulation in genes controlling fatty acid uptake and oxidation.50 In contrast, pressure-overload hypertrophy PPARα activity is reduced, leading to a down-regulation of genes controlling fat metabolism and in turn leading to an up-regulation of glucose use.51 These chronic adaptations can induce numerous detrimental effects that extend beyond alterations in energy production and may include enhanced oxygen free-radical production, impaired energetics, stimulation of apoptosis, and the induction of LV dysfunction. Discussed in the sections that follow is how metabolic imaging has helped characterize this loss of metabolic flexibility due to these chronic adaptations in various disease processes.
Gender and Aging
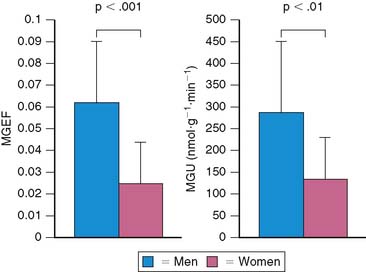
Both gender and aging impact the myocardial metabolic phenotype. Results of studies in animal models show that there are sex differences in myocardial substrate metabolism, with female rats exhibiting less myocardial glucose and more fatty acid metabolism.52,53 Recently, using PET with 11C-glucose and 11C-plamitate, these observations were confirmed in young healthy volunteers.54 Women exhibited lower levels of glucose metabolism compared with men (Fig. 40-6). Although no differences in myocardial fatty acid metabolism were noted, women also exhibited higher MVO2 compared with men, as measured by PET with 11C-acetate. These gender differences in substrate metabolism become more pronounced as one transitions to more pathologic conditions. For example in addition to the changes in glucose metabolism and MVO2, obese women exhibited higher fatty acid uptake and oxidation compared with obese men.55 The differences in myocardial metabolism could not be explained by differences in myocardial blood flow, insulin sensitivity, hemodynamics, myocardial work, or the plasma substrate environment. Differences in hormonal stimulation may play a role in the mechanism of sex-related differences in myocardial substrate metabolism, since results of animal studies have shown that estrogen decreases glucose oxidation, gluconeogenesis, and glycogenolysis and increases fatty acid oxidation in liver and skeletal muscle.56–59 Myocardial glucose utilization may also be decreased by estrogen; it is known to up-regulate nitric oxide synthases, which cause a reduction in GLUT4 translocation to the cell surface, thereby inhibiting myocardial glucose uptake.60–62 Indeed, in a small retrospective study, PET-derived measurements of myocardial fatty acid metabolism were higher in postmenopausal women, compared with either postmenopausal women not receiving hormonal replacement therapy or age-matched men consistent with a decline in glucose uptake.63
In various experimental models of aging, the contribution of fatty acid oxidation to overall myocardial substrate metabolism declines with age.64,65 It appears the cause for the decrease in fatty acid oxidation is multifactorial, ranging from changes in mitochondrial lipid content, oxygen free-radical injury, a decline in carnitine palmitoyltransferase-1 activity, and an age-related decline in myocardial PPARα activity.66–68 Using the PET approaches described above, it has been shown that a similar metabolic shift occurs in healthy older humans.69 However, despite this preference for glucose as an energy substrate, older individuals are unable to increase glucose utilization in response to β-adrenergic stimulation with dobutamine to the same extent as younger individuals. This impaired metabolic response may portend a stress-related energy deprivation state in the aging heart or potentially indicate that the heart is more susceptible to injury during periods of ischemia.70 Recently it has been shown that this impairment in metabolic reserve can be ameliorated by endurance exercise training in older subjects.71
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree