Chapter 31 Imaging in Patients Receiving Cardiotoxic Chemotherapy
INTRODUCTION
Of the many chemotherapeutic agents used in the treatment of malignancies, the anthracycline doxorubicin is well established for its therapeutic efficacy, despite its potential cardiotoxicity. Doxorubicin (Adriamycin) has been available for more than 30 years.1,2 Although a number of other anthracycline derivatives are available, doxorubicin is the most extensively used agent. Currently, it is used widely in the treatment of breast and gynecologic malignancies, lymphoma, and lung cancer. The cardiotoxicity associated with doxorubicin can be both acute and chronic.3 Acute cardiotoxicity is transient and without permanent consequences. It involves hypotension, pericarditis, tachycardia, or other arrhythmias. Chronic cardiotoxicity is of greater concern because it can cause progressive left ventricular dysfunction leading to clinical congestive heart failure, which in its most severe form can be fatal.
Some of the earliest clinical paradigms for nuclear cardiology techniques for monitoring and assessing therapeutic efficacy involved doxorubicin and its cardiotoxicity. Remarkably, techniques first reported in the late 1970s continue to be relevant.4 This chapter focuses on nuclear cardiology approaches to following patients receiving potentially cardiotoxic chemotherapy. It focuses on global left ventricular function monitoring, which is the most widely used clinical approach, and also discusses alternative nuclear and nonnuclear monitoring strategies.
MECHANISMS OF CARDIOTOXICITY
The most commonly accepted view of the mechanism of doxorubicin cardiotoxicity involves a combination of oxidative stress, or free radical production by the metabolites of doxorubicin, coupled with sensitivity of the myocardium to the cytotoxic effects of oxidative stress.5,6 Generated free radicals are usually toxic, reacting with a number of cell constituents and leading to lipid peroxidation, depletion of specific peptides, and damage to nucleic acids. In addition, mammalian myocardium, in contrast to other organs, is relatively deficient in enzymatic free radical scavengers that can potentially reverse the process. Doxorubicin metabolites also may have direct effects on these enzymes. In addition, doxorubicin can bind to cellular ionic iron, resulting in a complex that is highly toxic to intracellular proteins and membrane lipids.7
Alternative mechanisms involve calcium overload, release of vasoactive amines, decrease in cardiac muscle protein gene expression, and induction of apoptosis.8–12
PATHOLOGY
The myocardial damage associated with doxorubicin toxicity involves myofibrolysis, cytoplasmic vacuolization as a result of ballooning of sarcoplasmic reticulum, and degeneration of nuclei and mitochondria.13 Such changes ultimately result in substantial fibrosis. Minor grades of change are usually seen, even after lower doses. Higher grades are associated with impending congestive heart failure.14 A grading system for evaluating histopathologic changes based on the findings of endomyocardial biopsy has been described (Table 31-1). Intrinsic relationships between the severity of histopathologic abnormality and the degree of left ventricular dysfunction are poor. Even though there is an approximate linear relationship between histopathologic changes and cumulative dose, there is such marked variation that specific relationships cannot be readily established in individual patients.15 Further, sampling of biopsy material, because of the small sample size, may lead to inadequate identification of a more severe problem. On the other hand, the reverse can occur, and the degree of dysfunction can be overestimated based on a sampling error as a result of the nonuniform pathologic process.
Table 31-1 Histopathologic Grading of Doxorubicin Cardiotoxicity
| Grade 0 | No abnormality |
| Grade 1 | Minimal number of cells (<5% of total cells in each block) with early changes (early myofibrillar loss or distended sarcoplasmic reticulum) |
| Grade 1.5 | Small groups of cells involved (5%-15% of total number), some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 2 | Groups of cells involved 16%-25% of total number, some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 2.5 | Groups of cells involved 26%-35% of total number, some of which have definite changes (marked myofibrillar loss or cytoplasmic vacuolization) |
| Grade 3 | Diffuse cell damage (>35% of total number of cells) with marked changes (total loss of contractile elements, loss of organelles, mitochondrial and nuclear degeneration) |
Adapted from Billingham ME, Mason JW, Bristow MR, Daniels JR: Anthracycline cardiomyopathy monitored by morphologic changes, Cancer Treat Rep 62:865–872, 1978.
INCIDENCE OF CARDIOTOXICITY
Although the severity and occurrence of clinical cardiotoxicity are dose dependent, there is considerable variability in individual susceptibility. As a result, some patients may experience substantial left ventricular dysfunction at relatively lower doses, whereas others may tolerate high cumulative doses with minimal if any effects. Usually, doses of more than 450 to 500 mg/m2 result in increased occurrence of cardiotoxicity. Older studies reported an incidence of heart failure of approximately 2% at a cumulative dose of 300 mg/m2 or less and 7% at a dose of 550 mg/m2. The incidence rose to more than 20% at a cumulative dose of more than 700 mg/m2.16,17 No specific genetic or biochemical markers to predict susceptibility have been identified. However, retrospective analysis shows that certain clinical risk factors are associated with a predilection for doxorubicin cardiotoxicity, including antecedent cardiac disease, age, mediastinal radiation, concomitant cyclophosphamide therapy, concomitant paclitaxel therapy, and, more recently, concomitant therapy with monoclonal antibody against epidermal growth factor (HER2).3
CLINICAL COURSE
Left ventricular dysfunction may occur without identifiable cardiac symptoms before clinical congestive heart failure develops. Left ventricular dysfunction induced by doxorubicin is usually irreversible. However, in a significant number of patients, improvement (and even normalization) has occurred after discontinuation of doxorubicin therapy or institution of appropriate therapy for congestive heart failure.18–20 If treatment with doxorubicin is continued after asymptomatic left ventricular dysfunction occurs, a progressive and rapid decline in ventricular performance leading to the clinical manifestation of congestive heart failure is likely.20 It is unusual for patients who have completed a course of doxorubicin chemotherapy to have ventricular dysfunction and heart failure after discontinuation of therapy.11 However, this phenomenon is seen in a relatively small proportion of patients.
MONITORING FOR CARDIOTOXICITY
Radionuclide angiocardiography, initially by first-pass techniques and more recently by equilibrium techniques, remains the standard for assessing potential cardiotoxicity (see Chapter 12).19 Neither clinical examination nor electrocardiography or more standard clinical laboratory evaluation is efficacious in this regard. The use of radionuclide angiocardiography for measuring left ventricular ejection fraction as the functional index of cardiac performance was established in a variety of clinical settings. Although this measure is not a pure index of contractility and is influenced by preload and afterload, it has excellent accuracy and reproducibility.21 It can be performed in virtually all patients and is not limited by acoustic window artifacts that can affect ultrasound studies. The technique is fully automated22 and well standardized and provides the level of reproducible quantitative data necessary for reliable serial application.
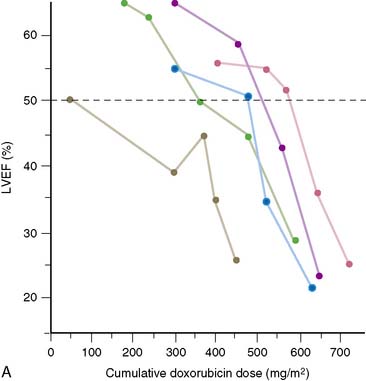
In 1979, Alexander and colleagues4 initially described 55 patients monitored with serial first-pass quantitative radionuclide angiocardiography at rest. They observed a significant relationship between the cumulative dose of doxorubicin and the development of ventricular dysfunction. Five patients in this series had heart failure; all five had an ejection fraction of less than 30% when symptoms occurred (Fig. 31-1). Based on this initial experience, guidelines were suggested.
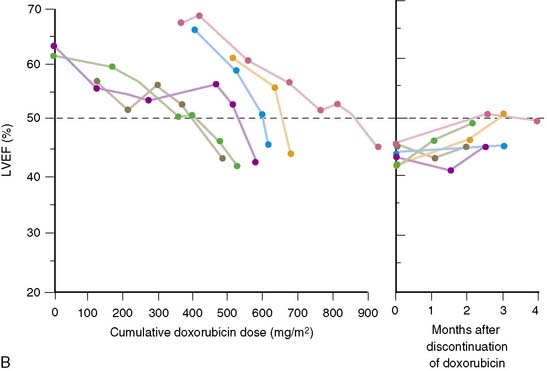
Schwartz and colleagues, nearly a decade later, reported a larger experience with the monitoring of 1487 patients over a 7-year period.19 Based on this experience, the initial guidelines were slightly amended. These guidelines remain in place today (Table 31-2). In patients with a normal baseline ejection fraction (>50%), moderate toxicity was defined to include an end point of a decline of more than 10% in absolute ejection fraction, with a final ejection fraction of less than 50%.
Table 31-2 Guidelines for Monitoring Doxorubicin Cardiotoxicity by Serial Radionuclide Angiography
| Baseline evaluation: Baseline radionuclide angiocardiography at rest is done to estimate left ventricular ejection fraction (LVEF) before the start of doxorubicin therapy or before 100 mg/m2 of doxorubicin has been given. |
| Subsequent evaluations: Subsequent studies are done 3 weeks after the indicated last dose of doxorubicin and before consideration of the next dose, at the following intervals: |
I. Patients with normal baseline LVEF (≥50%)
Get Clinical Tree app for offline access

|