As a quick reference to understanding the HRCT nomenclature used in this book, in this glossary, we have listed a number of useful HRCT terms, their definitions and significance, and in many cases have provided illustrations of their typical appearances. The terms defined and the definitions used largely incorporate all the recommendations of the Nomenclature Committee of the Fleischner Society, which were first published in 1984, were modified (1), and have recently been revised (1–3), but also reflect our personal preferences (4). Only the Fleischner-defined terms appropriate to the use of HRCT are reviewed in this glossary, and some terms not included in the most recent Fleischner Society glossary of thoracic imaging terms (3) are also defined, particularly when appropriate to HRCT diagnosis or used in this text.
As a general rule, HRCT terms are based on their specific associated anatomical correlates. Purely descriptive terms have been avoided, except in situations in which the findings themselves are nonspecific and cannot be related to particular anatomical abnormalities, when the nonspecific descriptive term is particularly helpful in understanding and recognizing the abnormal finding, or when the descriptive term is widely accepted.
Acinus
A unit of lung structure distal to a terminal bronchiole and supplied by first-order respiratory bronchioles. An acinus is the largest lung unit in which all airways participate in gas exchange. Acini average 7 to 8 mm in diameter in adults and range from 6 to 10 mm in diameter (Fig. 23-1) (5–9). Individual acini are not visible on HRCT in normal subjects, although acinar arteries can sometimes be seen. Secondary pulmonary lobules are comprised of a varying number of acini, ranging from 3 to 24 (10). Acinar opacification can sometimes be seen as a poorly marginated nodule (3), termed an acinar nodule.
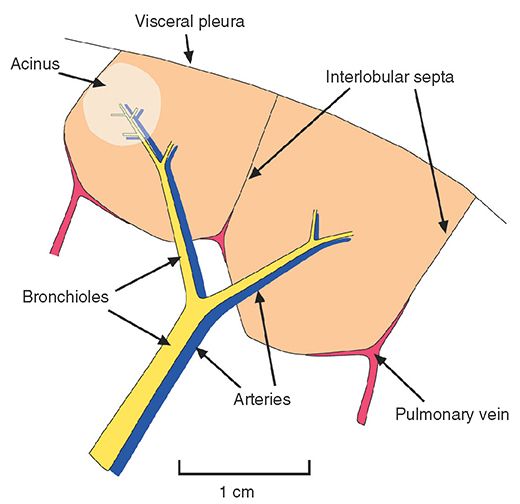
FIGURE 23-1 Anatomy of the pulmonary acinus relative to the secondary pulmonary lobule, as described by Miller.
Equivalent: pulmonary acinus
Acinus: Acinar Nodule
Acinar nodules are round or ovoid poorly defined pulmonary opacities approximately 5 to 8 mm in diameter, presumed to represent an anatomic acinus rendered opaque by consolidation. This term is used only in the presence of numerous such opacities (3). Acinar nodule is preferred to airspace nodule in the current Fleischner Society glossary, although the two terms can be considered to be equivalent. See airspace nodule.
Air Bronchogram
The appearance on radiographs or CT of an air-filled bronchus surrounded and outlined by abnormally dense lung, whether it is densely consolidated or of ground-glass opacity (Fig. 23-2) (3). The presence of an air bronchogram implies patency of proximal airways and a lung abnormality characterized by replacement of alveolar air.
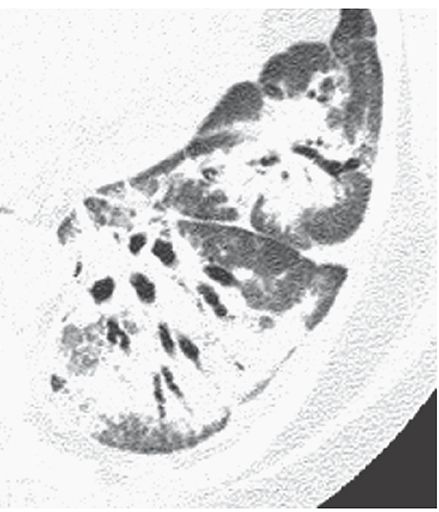
FIGURE 23-2 Airspace consolidation in organizing pneumonia. Air bronchograms are visible within the dense lower lobe.
Air Crescent Sign
An appearance on radiographs or CT, in which a crescent of air (Fig. 23-3) outlines the inner edge of a lung cavity and caps a rounded intracavitary mass or opacity (3). It may be seen with superinfection of a preexisting cyst (mycetoma), septic infarction of lung (e.g., angioinvasive aspergillosis), neoplasm, clot within a cavity, hydatid disease, and some other entities.
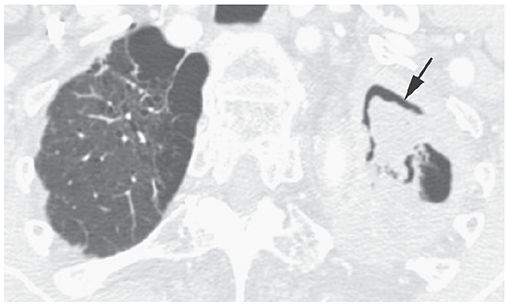
FIGURE 23-3 Air crescent sign (arrow) in the left upper lobe. This reflects the presence of a mycetoma.
Airspace
A term referring to an air-containing lung structure, including respiratory bronchioles but excluding purely conducting airways, such as terminal bronchioles (3).
Airspace Consolidation
See consolidation.
Airspace Nodule
A small, nodular opacity, usually ranging from a few millimeters to 1 cm in diameter seen in patients who have airspace diseases. It usually represents a focal area of peribronchiolar inflammation or airspace consolidation (11–14), although some such opacities may be acinar. Airspace nodules are typically ill-defined and often appear centrilobular in location (Fig. 23-4). HRCT findings are unreliable in distinguishing small nodules that are primarily airspace in origin from those that are primarily interstitial; thus, a description of the size, appearance, and distribution of nodules is usually more appropriate when interpreting HRCT. See centrilobular and nodule.
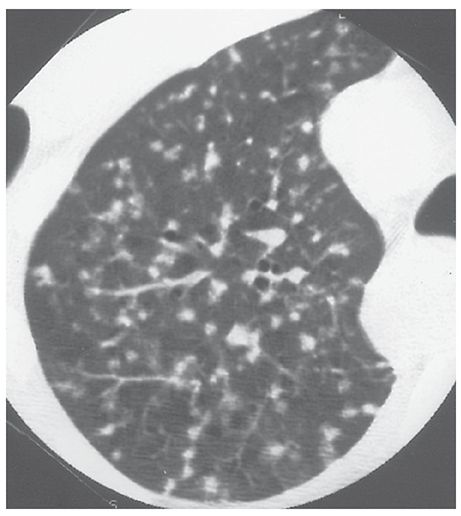
FIGURE 23-4 Airspace nodules. In this patient who has endobronchial spread of tuberculosis, small, ill-defined, centrilobular nodules reflect the presence of peribronchiolar inflammation and consolidation. As is typical of centrilobular nodules, some are associated with small arteries, and many are centered 5 to 10 mm from the pleural surfaces.
This term is largely equivalent to “acinar nodule,” which is presumed to represent an opacified lung acinus.
Air Trapping
Abnormal retention of air (i.e., gas) within a lung or part of a lung, especially during or after expiration, as a result of airway obstruction or abnormalities in lung compliance. It is diagnosable if lung parenchyma remains lucent on postexpiratory CT scans, shows a less than normal increase in attenuation after expiration, or shows little change in cross-sectional area (Fig. 23-5) (1,15–22). Air trapping is difficult to diagnose with certainty on inspiratory scans; lung inhomogeneity on inspiratory scans in patients who have airways disease should usually be referred to as mosaic perfusion or mosaic attenuation.
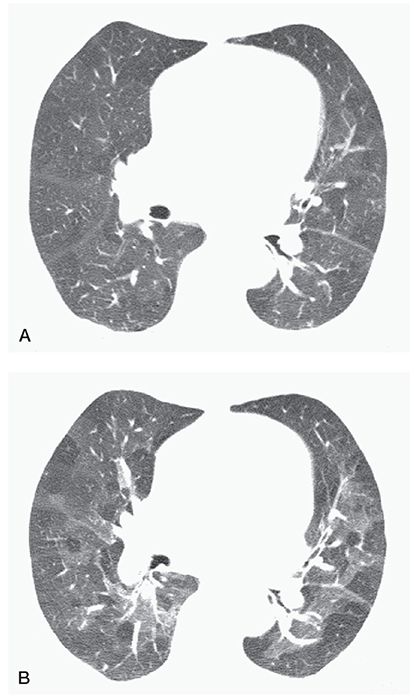
FIGURE 23-5 Air trapping in a patient with postinfectious bronchiolitis obliterans. A: On inspiration, the lungs appear heterogeneous in attenuation because of mosaic perfusion. B: On expiration, marked inhomogeneity in lung attenuation is noted, with multifocal air trapping.
Equivalent: gas trapping
Architectural Distortion
A manifestation of lung disease in which abnormal displacement of pulmonary structures, including bronchi, vessels, fissures, and interlobular septa, results in a distorted appearance of lung anatomy (1,3,4). This finding is commonly seen in the presence of fibrosis or volume loss.
Atelectasis
A reduction in lung volume or inflation, either localized or involving an entire lung, and often associated with an increase in lung opacity or attenuation. It may occur because of resorption of gas distal to an obstructing lesion, lung compression, deficiency of surfactant, or fibrosis.
Synonyms: collapse, volume loss
Atoll Sign
See reversed halo sign.
Beaded Septum Sign
Nodular thickening of interlobular septa that suggests the appearance of a row of beads (Fig. 23-6). This finding is most common in patients who have lymphangitic spread of neoplasm (23) and sarcoidosis, but overall, is an uncommon HRCT finding, even in these diseases.
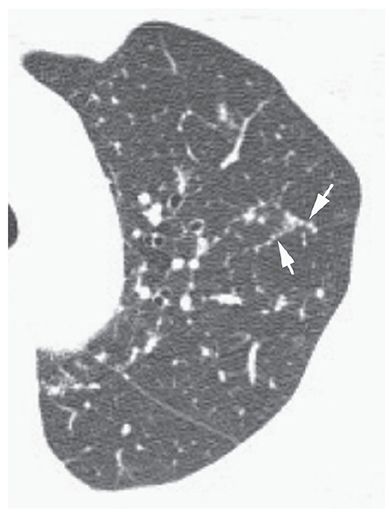
FIGURE 23-6 Beaded septum sign. In a patient with sarcoidosis, interlobular septa in the upper lobe are nodular or beaded in appearance (arrows).
Bleb
A gas-containing space within the visceral pleura (2). Radiologically, this term is sometimes used to describe a focal thin-walled lucency, contiguous with the pleura, usually at the lung apex. However, a distinction between bleb and bulla is of little practical significance and is seldom justified (3). On HRCT, a bleb and bulla cannot be distinguished, and the term “bulla” is usually preferred (2,3).
Bronchiectasis
Localized or diffuse, irreversible bronchial dilatation, usually resulting from chronic infection, airway obstruction by tumor, stricture, impacted material, inherited bronchial abnormalities, or fibrosis (see traction bronchiectasis). Although the definition of this term indicates that abnormalities must be irreversible, this is difficult to establish in the absence of serial examinations and is not required for diagnosis. Bronchiectasis can be classified into three types (cylindrical, varicose, and cystic), depending on the appearances of the abnormal bronchi (Figs. 23-7 and 23-8). Although bronchial dilatation is the primary feature of bronchiectasis, bronchial wall thickening, fluid retention within the bronchi, and small airway abnormalities are also commonly visible on HRCT.
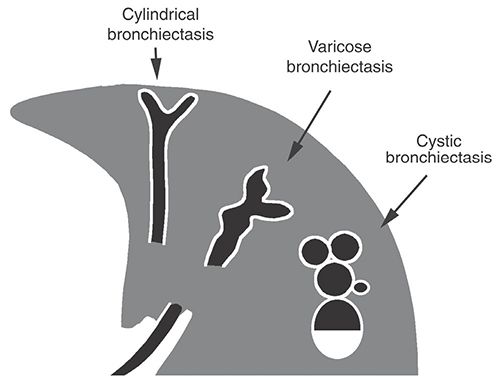
FIGURE 23-7 Bronchiectasis. Classification of bronchiectasis based on morphology and HRCT appearance.
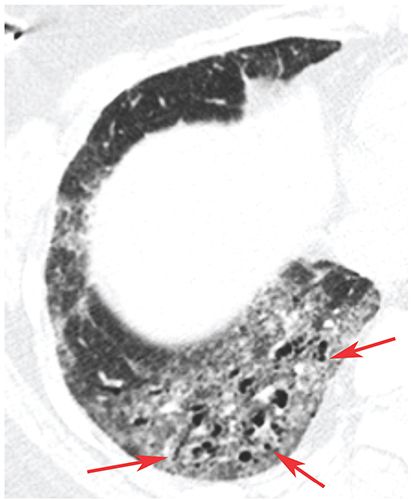
FIGURE 23-8 Bronchiolectasis. In a patient with scleroderma and fibrotic NSIP, there is ground-glass opacity and dilated air-filled bronchioles in the lung periphery (arrows), so-called traction bronchiolectasis.
Bronchiole
Airways not containing cartilage in their walls are termed “bronchioles.” The largest bronchioles measure approximately 3 mm in diameter and have walls approximately 0.3 mm in thickness. Secondary lobules are supplied by arteries and bronchioles measuring approximately 1 mm in diameter, whereas intralobular terminal bronchioles and arteries measure approximately 0.7 mm in diameter, and acinar bronchioles and arteries range from 0.3 to 0.5 mm in diameter (Fig. 23-1). Bronchioles are not normally visible on HRCT.
Bronchiolar Impaction
See bronchiolectasis and tree–in–bud sign.
Bronchiolectasis
Dilatation of bronchioles. Bronchiolectasis can occur as a result of airways disease or the presence of lung fibrosis (see traction bronchiolectasis) (Fig. 23-8). Dilated bronchioles can be air or fluid filled. Dilated fluid-filled bronchioles are often described using the terms “bronchiolar impaction” or “tree-in-bud” (24–28), or may be visible as centrilobular nodular opacities.
Bronchiolitis
Bronchiolar inflammation, either infectious or noninfectious. It may be associated with centrilobular (i.e., peribronchiolar or bronchiolocentric) nodules, tree-in-bud, or obstruction with mosaic perfusion and air trapping. Pathologically, bronchiolitis is often described as “cellular” or “constrictive.”
Bronchocentric
An abnormality that occurs in relation to or surrounding bronchi may be described using this term.
Bronchovascular Bundle
See peribronchovascular interstitium.
Bulla
A sharply demarcated area of emphysema, measuring 1 cm or more in diameter and possessing a wall less than 1 mm in thickness (Fig. 23-9) (2). To make the diagnosis of a bulla on HRCT, other areas of emphysema should also be visible. Subpleural bullae are commonly the result of paraseptal emphysema (29–31).
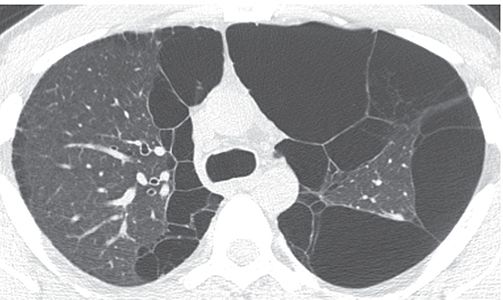
FIGURE 23-9 Bullae. Large thin-walled subpleural bullae are visible bilaterally, in association with centrilobular emphysema. Because of the predominance of bullae in this patient, the term “bullous emphysema” could be used to describe this appearance.
Bullous Emphysema
Emphysema in which bullae are the predominant feature (Fig. 23-9) (32).
Cavity
A cavity is an air-filled space, seen as a lucency within an area of pulmonary consolidation, mass, or nodule. A cavity is usually produced by the expulsion or drainage of a necrotic part of the pathologic lesion via the bronchial tree. It sometimes contains a fluid level (3). A thin-walled air-filled space should not be referred to as a “cavity” unless it is known that cavitation, that is, necrosis and expulsion, has occurred.
Cavity is not a synonym for abscess or cyst.
Centrilobular
An adjective describing a structure (e.g., centrilobular bronchiole), HRCT finding (e.g., centrilobular nodule), or disease process that involves the center of the lobule. It is also used to describe abnormal findings seen in relation to centrilobular structures such as bronchioles or small arteries that cannot be precisely localized to the centers of lobules (Figs. 23-4 and 23-10). On HRCT, a centrilobular abnormality can appear as an opacity or lucency centered within the lobule or as a group of opacities or lucencies surrounding centrilobular arteries (11,24,25). It can reflect inflammation, airspace consolidation, airways disease, interstitial fibrosis, or emphysema.
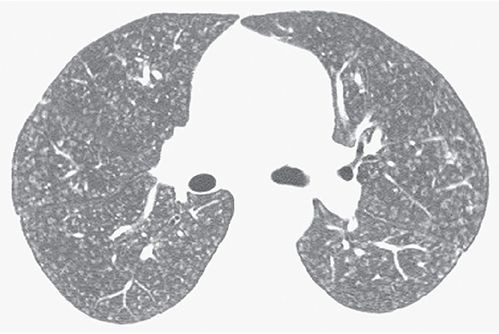
FIGURE 23-10 Ill-defined centrilobular nodules in a patient with hypersensitivity pneumonitis. The nodules are separated from the pleural surfaces and fissures by a distance of several millimeters.
Equivalent: lobular core
Centrilobular Emphysema
Emphysema that predominantly affects the respiratory bronchioles in the center of acini and, therefore, predominantly involves the central portion of secondary lobules (29–31). It is common in the upper lobes and in smokers. It is usually visible on HRCT as multifocal areas of lucency that lack visible walls, although thin walls can sometimes be seen (Fig. 23-11). Occasionally, the lucencies can be seen to surround the centrilobular artery.
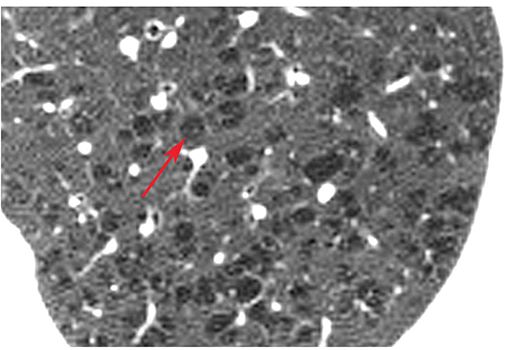
FIGURE 23-11 Centrilobular emphysema. Detailed view of the left upper lobe in a patient with centrilobular emphysema. Numerous focal rounded lucencies (arrow) without walls are visible. This appearance is typical of centrilobular emphysema. The lucent areas of emphysema are located in the centers of lobules, and surround the centrilobular artery.
Equivalents: centriacinar emphysema, proximal acinar emphysema
Centrilobular Interstitial Thickening
Thickening of the centrilobular peribronchovascular interstitium that surrounds centrilobular bronchioles and vessels. It is recognizable by an increase in prominence of centrilobular structures.
Centrilobular Interstitium
The peripheral, centrilobular extension of the peribronchovascular interstitium. Part of the axial fiber network described by Weibel (6).
Equivalents: centrilobular peribronchovascular interstitium, axial interstitium (2)
Centrilobular Structures
Structures in the center of pulmonary lobules, most notably, the centrilobular bronchioles and arteries. Centrilobular arteries and their immediate branches measure approximately 1 mm and 0.5 to 0.7 mm in diameter, respectively (1,33), and are normally visible on HRCT. Centrilobular bronchioles have a wall measuring approximately 0.15 mm in thickness and are not normally visible on HRCT.
Conglomerate Mass
A large opacity that often surrounds and encompasses bronchi and vessels, usually in the central or perihilar lung (Fig. 23-12). It often represents a mass of fibrous tissue or confluent nodules. It is most common in silicosis, coal worker’s pneumoconiosis, and sarcoidosis (Fig. 23-12).
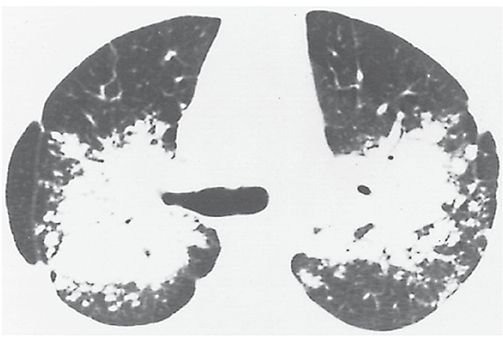
FIGURE 23-12 Conglomerate mass. Bilateral upper lobe masses obscuring the perihilar vessels in a patient who has sarcoidosis. Air bronchograms are visible within the masses. These conglomerate masses reflect a multitude of confluent granulomas. This appearance could also be referred to as consolidation.
Equivalents in pneumoconiosis patients: complicated pneumoconiosis, progressive massive fibrosis
Consolidation
An increase in lung opacity, discernible on plain radiographs or HRCT, that results in obscuration of underlying vessels (Figs. 23-2, 23-12, and 23-13). This finding usually indicates the replacement of alveolar air or filling of airspaces by fluid, cells, or tissue, but can also be seen with extensive interstitial disease. On HRCT, it should be differentiated from ground-glass opacity, in which underlying vessels are not obscured by increased lung opacity (3).
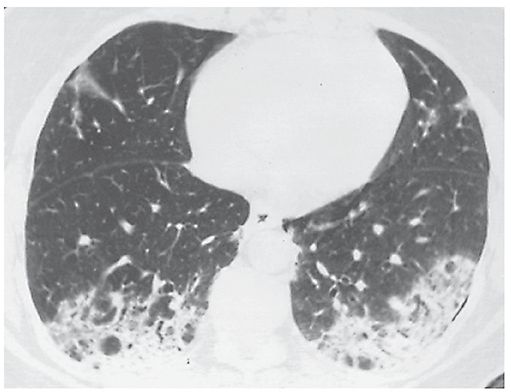
FIGURE 23-13 Consolidation. A patient who has bronchiolitis obliterans organizing pneumonia shows bilateral consolidation. Pulmonary vessels are invisible in the densest areas.
Equivalents: airspace consolidation, airspace opacification, airspace attenuation
Crazy-Paving Pattern
The superimposition of ground-glass opacity and a reticular pattern, often having the appearance of interlobular septal thickening (34–36). This pattern was first recognized in patients who had pulmonary alveolar proteinosis (PAP) (37) (Fig. 23-14) and is quite typical of PAP, but may also be seen in patients who have a variety of other diseases. In patients who have crazy paving, ground-glass opacity may reflect the presence of airspace or interstitial abnormalities (35,36); the reticular opacities may represent interlobular septal thickening, thickening of the intralobular interstitium, irregular areas of fibrosis, or a preponderance of an airspace filling process at the periphery of lobules or acini (35).
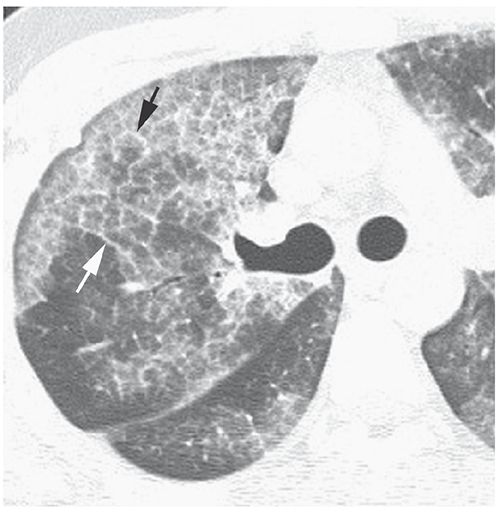
FIGURE 23-14 Crazy paving in pulmonary alveolar proteinosis. The abnormal lung regions show a combination of patchy ground-glass opacity and interlobular septal thickening (arrows).
Cyst
A nonspecific term describing the presence of a thin-walled (usually less than 2- or 3-mm thick), well-defined and circumscribed, air- or fluid-containing lesion, 1 cm or more in diameter that has an epithelial or fibrous wall (Fig. 23-15) (2–4). On HRCT, the term “cyst” is usually used to refer to an air-containing lesion or air-filled cyst. Air-filled cysts are commonly seen in patients who have Langerhans histiocytosis, lymphangiomyomatosis, sarcoidosis, and lymphoid interstitial pneumonia (38–42), but can be seen in other diseases as well. Honeycombing also results in the presence of cysts (i.e., honeycomb cyst). The term “cyst” can be used to describe the dilated bronchi seen in patients who have cystic bronchiectasis (Fig. 23-7), although the latter term is preferred. This term is not used to refer to focal lucencies associated with emphysema; instead, “bulla” is the preferred term.
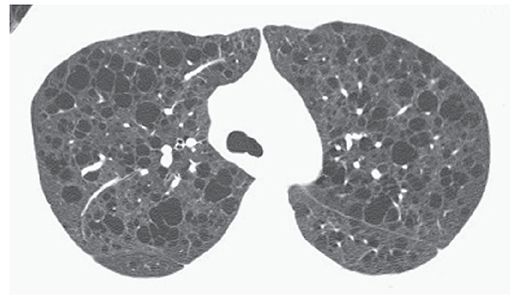
FIGURE 23-15 Multiple lung cysts in a patient who has lymphangiomyomatosis. The numerous low attenuation air-filled cysts are marginated by thin walls.
Cystic Airspace
An enlarged airspace surrounded by a wall of varying thickness, which may be thin, as in emphysema or lymphangiomyomatosis, or thick, as in honeycombing. See also bulla, cyst, honeycomb cyst, and pneumatocele.
Dependent Opacity
An ill-defined subpleural opacity, ranging from a few millimeters to 1 cm or more in thickness, that is only visible in dependent lung regions and disappears when the lung region is nondependent (Fig. 23-16) (2,43). Dependent opacity represents normal dependent atelectasis. It is visible in the posterior lung when the subject is supine and disappears in the prone position. This normal finding may also appear as a subpleural line, although this term is best used to refer to a thin, sharply defined opacity that persists in the nondependent lung.
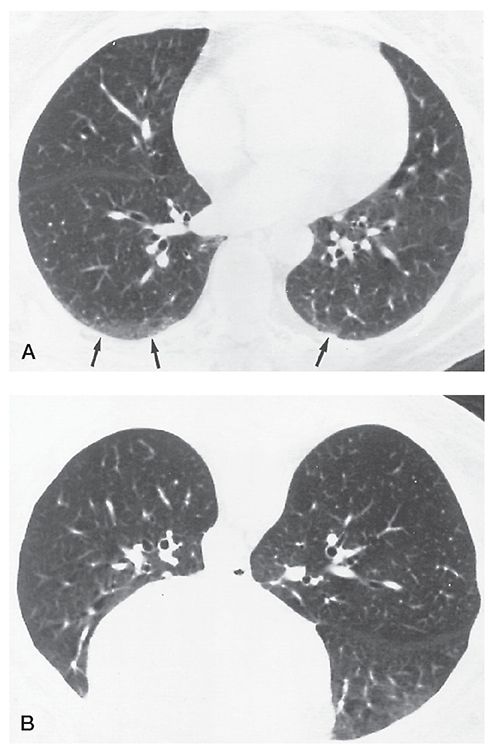
FIGURE 23-16 Dependent opacity. A: Ill-defined opacities (arrows) are visible in the posterior lungs, more evident on the right than the left. B: At the same level with the patient positioned prone, the posterior lungs appear normal.
Equivalent: dependent increased attenuation
Distal Acinar Emphysema
See paraseptal emphysema.
Dynamic Expiratory HRCT
HRCT scans performed during expiration to diagnose air trapping or airway collapse (15,16,20,44).
Emphysema
Permanent, abnormal enlargement of airspaces distal to the terminal bronchiole, accompanied by the destruction of their walls (29). Previous definitions of emphysema have included the caveat “without obvious fibrosis” (30), but recent observations have established that some associated fibrosis is not uncommon (29). Visible on HRCT as areas of low attenuation, usually without visible walls, and classified morphologically relative to the pulmonary lobule as centrilobular, panlobular (see Fig 23-28), or paraseptal (Fig. 23-17) (33,45). See also bulla, bullous emphysema (Fig. 23-9), centrilobular emphysema (Fig. 23-11), irregular airspace enlargement, panlobular emphysema, and paraseptal emphysema.
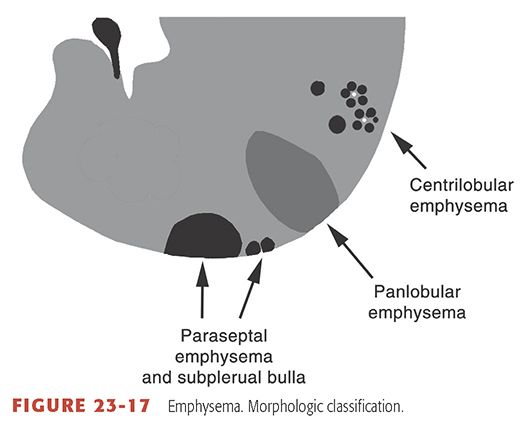
FIGURE 23-17 Emphysema. Morphologic classification.
End-Stage Lung
A term not commonly used. The final stage in progression of a lung disease, usually characterized by fibrosis, alveolar dissolution, bronchiolectasis, and disruption of normal lung architecture. Generally speaking, end-stage lung is considered to be present in patients who have morphologic evidence of honeycombing, extensive cystic changes, or conglomerate fibrosis (46–48). Also see honeycombing and honeycomb lung.
Expiratory HRCT
HRCT scans performed during or after expiration to diagnose air trapping in patients who have obstructive lung disease (Fig. 23-5) (15,17,20,22). Scans can be obtained after expiration, can be gated to spirometry (49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


