IMPORTANT TOPICS
High-Resolution Computed Tomography Findings
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
CT Imaging Techniques for Evaluating Patients with COPD
Abbreviations Used in This Chapter | |
BI | bulla index |
COPD | chronic obstructive pulmonary disease |
DLCO | carbon monoxide diffusing capacity |
EBV | endobronchial valve |
FEV1 | forced expiratory volume in 1 second |
FVC | forced vital capacity |
GOLD | Global Initiative for Chronic Obstructive Lung Disease |
HU | Hounsfield units |
LVRS | lung volume reduction surgery |
MinIP | minimum-intensity projection |
MIP | maximum-intensity projection |
MLD | mean lung density |
PFT | pulmonary function test |
QCT | quantitative computed tomography |
TLC | total lung capacity |
VC | vital capacity |
EMPHYSEMA
As defined by the American Thoracic Society, emphysema is “a condition of the lung characterized by permanent, abnormal enlargement of airspaces distal to the terminal bronchiole, accompanied by the destruction of their walls” (1–3).
This definition also includes the caveat “without obvious fibrosis” (4). However, it is now clear that microscopic fibrosis is frequently identified accompanying centrilobular and paraseptal emphysema, also the consequence of cigarette smoking (5–7). In one histologic study, a focal usual interstitial pneumonia pattern was identified in 125 of 587 (21.3%) smokers (8). A similar, though less frequent, finding of mild lung fibrosis has also been reported, occurring in approximately 5% of patients in one study of patients with chronic obstructive pulmonary disease (COPD) (9). Further complicating the issue is the fact that fibrosis can also be seen in association with other smoking-related diseases, including respiratory bronchiolitis-interstitial lung disease and desquamative interstitial pneumonitis. There is also evidence to suggest that fibrotic nonspecific interstitial pneumonitis may also be related to cigarette smoking (10). As will be discussed in detail in the section on COPD, in select cases, the extent of lung fibrosis accompanying emphysema is of sufficient magnitude to warrant separate categorization as “combined pulmonary fibrosis and emphysema” (11).
Pathogenesis
The sequence of events leading to the development of emphysema in smokers is controversial (12). What is agreed upon, however, is that inhaled tobacco smoke attracts macrophages to distal airways and alveoli. Macrophages, in turn, along with airway epithelial cells, release chemotactic substances that serve to attract neutrophils and induce them to release elastases and other proteolytic enzymes (13,14). Macrophages also release proteases in response to tobacco smoke. These elastases have the ability to cleave a variety of proteins, including collagen and elastin. Lung elastin is normally protected from excessive elastase-induced damage by alpha-1-protease inhibitor (alpha-1-antiprotease or antitrypsin) and other circulating antiproteinases. However, tobacco smoke tends to interfere with the function of alpha-1-antiprotease. In combination, these interactions result in structural damage to the distal airways and alveoli in smokers, leading to emphysema. Inherited deficiency in alpha-1-antiprotease may similarly lead to lung destruction and emphysema.
Long accepted as the primary sequence of events leading to distal airspace destruction described earlier, emphysema is now considered by some to be the result of a complex interaction among “oxidative stress, inflammation, extracellular matrix proteolysis, and apoptotic and autophagic cell death” (15).
Recently, it has been shown that the initial abnormality in patients with emphysema is narrowing and disappearance of small conducting airways (16,17). In a series of reports of patients with the emphysema phenotype of COPD, including the use of micro-CT techniques, these authors have documented a 10-fold reduction in the number of terminal bronchioles and a 100-fold decrease in the total cross-sectional area of terminal bronchioles, in both emphysematous and nonemphysematous lung regions, leading the authors to speculate that changes in the terminal airways may be the primary abnormality leading to the development of emphysema (16,17).
Classification of Emphysema
Emphysema is usually classified into three main subtypes, based on the anatomical distribution of the areas of lung destruction, but the names applied to these subtypes by different investigators often differ (3,18). These subtypes are (a) proximal acinar, centriacinar, or centrilobular emphysema; (b) panacinar or panlobular emphysema; and (c) distal acinar or paraseptal emphysema. Although from an anatomical or pathologic point of view it is most appropriate to refer to these types of emphysema relative to the presence and type of acinar abnormalities (i.e., proximal acinar, panacinar, distal acinar), from the standpoint of understanding the use of high-resolution computed tomography (HRCT), it is more appropriate to refer to them relative to the way in which we perceive them at the lobular level (i.e., centrilobular, panlobular, paraseptal). As indicated in Chapter 2, acini cannot be resolved on HRCT. In the remainder of this chapter, the terms centrilobular, panlobular, and paraseptal are used to describe these three types of emphysema.
Centrilobular emphysema (proximal acinar emphysema, centriacinar emphysema) predominantly affects the respiratory bronchioles in the central portions of acini and therefore involves the central portion of the lobule. Panlobular emphysema (panacinar emphysema) involves the components of the acinus more or less uniformly and therefore involves the entire lobule. Paraseptal emphysema (distal acinar emphysema) predominantly involves alveolar ducts and sacs, with peripheral areas of lung destruction often marginated by interlobular septa.
Centrilobular emphysema usually results from cigarette smoking. It mainly involves the upper lung zones. In contrast, panlobular emphysema is classically associated with alpha-1-protease inhibitor (alpha-1-antitrypsin) deficiency, although it may also be seen without protease deficiency in smokers, in the elderly, distal to bronchial and bronchiolar obliteration, and associated with drug use (4). Paraseptal emphysema can be an isolated phenomenon in young adults, often associated with spontaneous pneumothorax, or can be seen in older patients with centrilobular emphysema (4,19). In their early stages, these three forms of emphysema can be easily distinguished morphologically. However, as they become more severe, their distinction becomes more difficult.
Bullae can develop in association with any type of emphysema, but they are most common with paraseptal or centrilobular emphysema. A bulla, by definition, is a sharply demarcated area of emphysema measuring 1 cm or larger in diameter and possessing a wall less than 1 mm in thickness (20). In some patients who have emphysema, bullae can become quite large, resulting in significant compromise of respiratory function; this syndrome is sometimes referred to as bullous emphysema. Bullae have been classified by Reid according to their location and the type of emphysema with which they are associated (21). According to this classification, type 1 bullae are subpleural in location and occur in patients who have paraseptal emphysema; type 2 bullae are also subpleural but are associated with generalized emphysema (centrilobular or panlobular); and type 3 bullae are associated with generalized emphysema, but occur within the lung parenchyma rather than in a subpleural location.
Irregular airspace enlargement is an additional type of emphysema occurring in patients who have pulmonary fibrosis; this form of emphysema is also referred to as paracicatricial or irregular emphysema (4,18).
Radiographic Findings
Radiographic abnormalities in patients who have emphysema generally reflect increased lung volume, lung destruction (reduced vascularity or bullae), or both (22–27). When both findings are used as criteria for diagnosis, sensitivity as high as 80% has been reported for chest films (24), although the likelihood of a positive diagnosis depends on the severity of disease (3). When only findings of lung destruction are used for diagnosis, plain films are only 40% sensitive (27). Although the accuracy of chest radiographs in diagnosing emphysema is somewhat controversial, it can be reasonably concluded from the studies performed that moderate to severe emphysema can be diagnosed radiographically, whereas mild emphysema is difficult to detect.
The presence of increased lung volume, or overinflation, can be very important in making the diagnosis of emphysema on plain radiographs, but overinflation is an indirect sign of this disease. Findings of increased lung volume are nonspecific and can be lacking in some patients who have emphysema while being present in patients who have other forms of obstructive pulmonary disease. A number of plain radiographic findings of overinflation have been validated in patients who have COPD, although their sensitivity and specificity vary. These include (a) a lung height of 29.9 cm or more, measured from the dome of the right diaphragm to the tubercle of the first rib; (b) flattening of the right hemidiaphragm on the lateral projection, with a height of less than 2.7 cm measured from anterior to posterior costophrenic angles; (c) flattening of the right hemidiaphragm on a posteroanterior radiograph, with the highest level of the dome of the right hemidiaphragm less than 1.5 cm above a perpendicular line drawn between the costophrenic angle laterally and the vertebrophrenic angle medially; (d) an increased retrosternal airspace, measuring more than 4.4 cm at a level 3 cm below the manubrial-sternal junction; and (e) the right hemidiaphragm at or below the level of the anterior end of the seventh rib (22–24,26,28–30).
The presence of bullae on chest radiographs is the only specific sign of emphysema, but this finding is infrequent and may not reflect the presence of generalized disease. A reduction in size of pulmonary vessels or vessel tapering can also reflect lung destruction, but this finding lacks sensitivity and can be unreliable.
High-Resolution Computed Tomography Findings
On HRCT, emphysema is characterized by the presence of areas of abnormally low attenuation, which can be easily contrasted with surrounding normal lung parenchyma if sufficiently low window means (–600 to –800 Hounsfield units [HU]) are used (31–33). In most instances, focal areas of emphysema can be easily distinguished from lung cysts or honeycombing; focal areas of emphysema often lack distinct walls (32–34).
Although various CT findings of increased lung volume may also be seen in patients who have COPD and emphysema (35), their identification is usually secondary to the more direct observation of lung destruction characteristic of the various types of emphysema. In a study of 74 patients (44 who had normal lung function and 30 who had COPD), significant correlations were observed between forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) and the tracheal index (transverse/anteroposterior diameter: r = 0.578, p < 0.0001), anteroposterior/transverse thoracic diameters at the tracheal carina (r = –0.523, p < 0.0001) and 5 cm below (r = –0.533, p < 0.0001), and lung bulging in the intercostal spaces (r = –0.462, p < 0.0001) (35).
Centrilobular Emphysema
Centrilobular emphysema of mild to moderate degree is characterized on HRCT by the presence of multiple small, polka-dot-like, round areas of low attenuation, several millimeters to a centimeter in diameter, distributed throughout the lung, but usually having upper lobe predominance. Areas of lucency often appear to be grouped near the centers of secondary pulmonary lobules, and may be seen to surround centrilobular artery branches, visible as round or branching opacities (Figs. 6-17 to 6-19 and 20-1 to 20-3, Table 20-1) (32,33,36–38). These lucencies correspond to the well-circumscribed centrilobular or centriacinar areas of lung destruction seen pathologically in patients who have centrilobular emphysema (32,33,37–40). Although the centrilobular location of lucencies cannot always be recognized on CT or HRCT (33,37,38), the presence of multiple small areas of emphysema scattered throughout the lung is diagnostic of centrilobular emphysema (Figs. 20-2 and 20-3). In most cases, the areas of low attenuation lack visible walls (34), although very thin and relatively inconspicuous walls are occasionally seen on HRCT (Figs. 20-4 and 20-5), probably related to surrounding fibrosis; it has been shown that centrilobular emphysema is commonly associated with some fibrosis (1,2). In patients who have centrilobular emphysema, bullae within the lung may have visible walls, and paraseptal emphysema and subpleural bullae are often seen (Figs. 20-4 to 20-7).
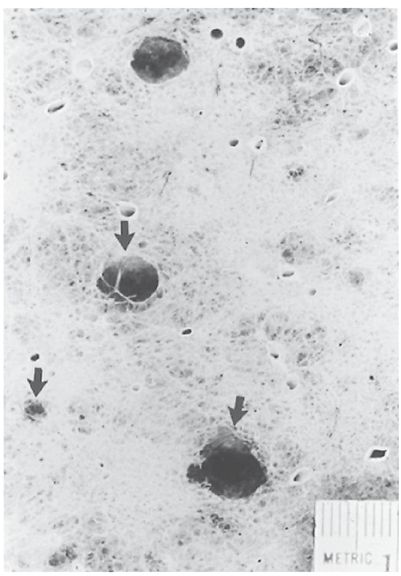
FIGURE 20-1 Centrilobular (centriacinar) emphysema. A low-power microscopic view of a lung specimen from a patient with mild centrilobular emphysema. Areas of lung destruction measuring 3 to 10 mm in diameter are visible (arrows).
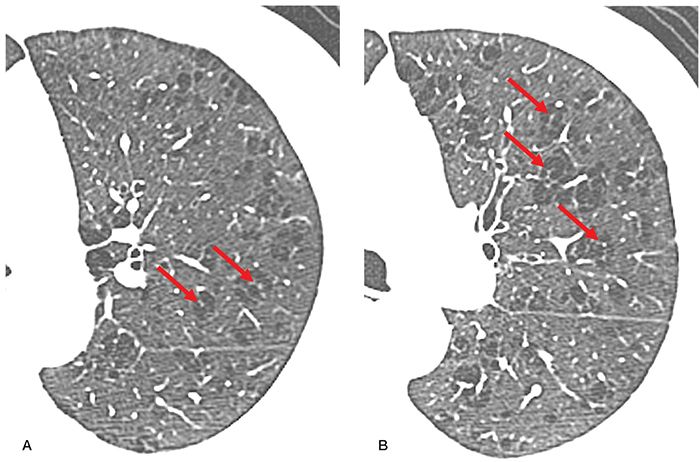
FIGURE 20-2 Centrilobular emphysema in a cigarette smoker. A and B: Targeted HRCT images through the left lung at the level of the aortic arch and left main pulmonary artery, respectively, show localized areas of low attenuation measuring 2 to 20 mm in diameter, which can be seen to be centrilobular, surrounding centrilobular arteries (arrows). These are identifiable either as central dots or branching structures. Note that the areas of destruction lack recognizable walls.
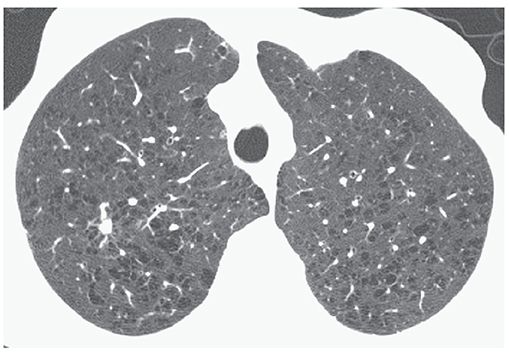
FIGURE 20-3 Centrilobular emphysema with small focal lucencies. Lucencies lack visible walls, as is common in patients who have centrilobular emphysema. Some lucencies can be seen to surround small vessel branches. This appearance indicates that the emphysema has a centrilobular location. A small nodule in the right upper lobe represents an adenocarcinoma.
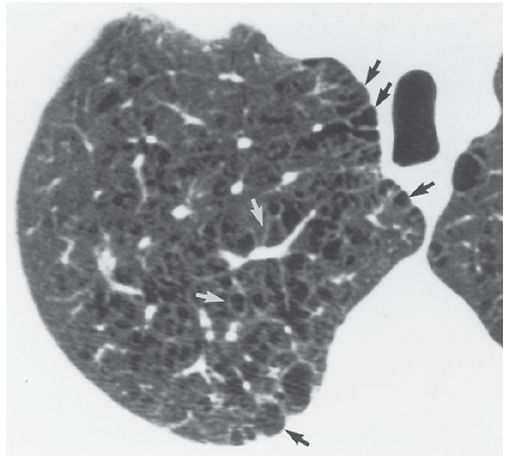
FIGURE 20-4 Centrilobular emphysema. Some of the focal areas of lung destruction appear to be outlined by very thin walls (white arrows), probably due to fibrosis. Subpleural lucencies (black arrows) represent paraseptal emphysema, which can coexist with centrilobular emphysema.
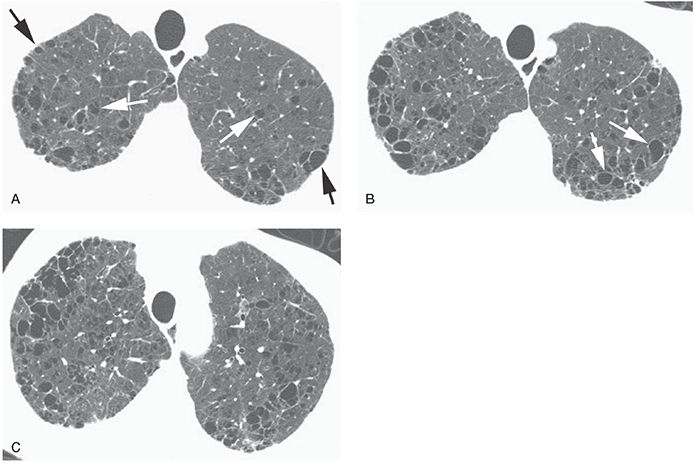
FIGURE 20-5 Centrilobular and paraseptal emphysema. A: Areas of centrilobular emphysema (white arrows) are seen as focal lucencies in the central lung, without visible walls. Paraseptal emphysema in the subpleural regions (black arrows) is also visible, and thin walls are typically visible on HRCT. B: At a lower level, small bullae (white arrows) are associated with centrilobular emphysema. These areas of emphysema show thin walls. C: At a level below B, centrilobular emphysema, intraparenchymal bullae, and paraseptal emphysema are all visible.
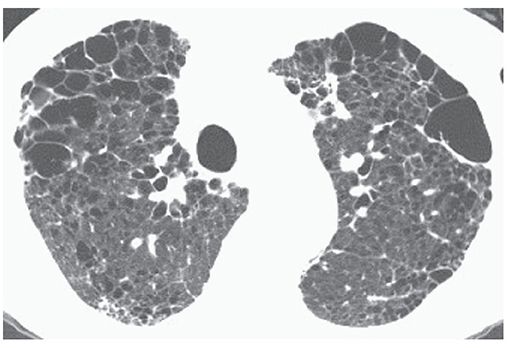
FIGURE 20-6 Centrilobular emphysema with extensive paraseptal emphysema. Subpleural bullae and bullae within the lung parenchyma are both associated with centrilobular emphysema in this patient.
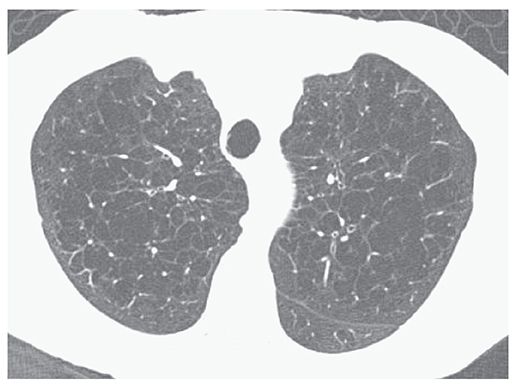
FIGURE 20-7 Confluent centrilobular emphysema. HRCT images through the upper lobes show confluent foci of emphysema in both lungs.
TABLE 20-1 HRCT Findings in Centrilobular Emphysema
Multiple, small, spotty, or centrilobular lucenciesa,b |
Upper lobe predominancea,b |
Lucencies without visible wallsa,b |
Thin walls visible in some |
Lucencies surrounding centrilobular arteriesb |
May be associated with paraseptal emphysema or bullae |
aMost common findings.
bFindings most helpful in differential diagnosis.
With more severe centrilobular emphysema, areas of destruction can become confluent. When this occurs, the centrilobular distribution of abnormalities is no longer recognizable on HRCT, or pathologically; the term confluent centrilobular emphysema is sometimes used to describe this occurrence (Figs. 6-21, 6-22, 20-7, 20-8, and 20-9). This appearance can closely mimic the appearance of panlobular emphysema, and a distinction between these is of little clinical significance.
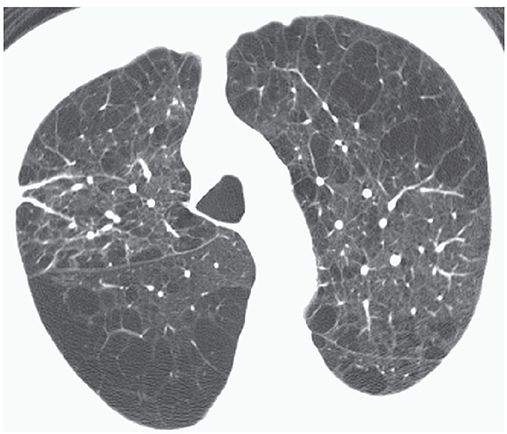
FIGURE 20-8 Confluent centrilobular emphysema. Areas of centrilobular and paraseptal emphysema are associated with extensive areas of lung destruction.
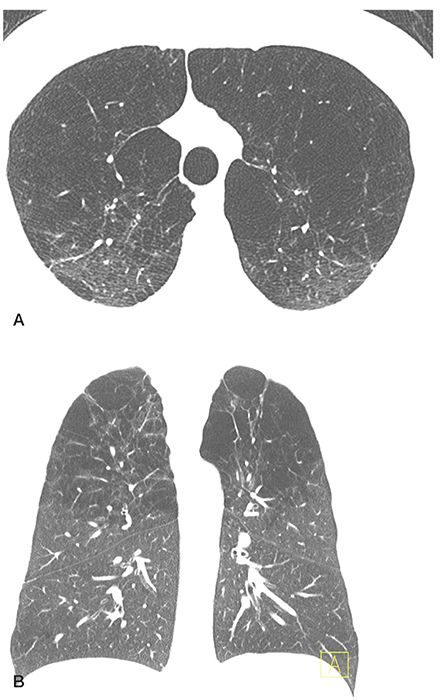
FIGURE 20-9 Confluent centrilobular emphysema. A and B: HRCT images at the level of the upper lobes and a coronal reconstruction, respectively, show areas of centrilobular and paraseptal emphysema are associated with extensive areas of lung destruction. This appearance mimics that of panlobular emphysema, but it is somewhat more patchy and is associated with a distinct upper lobe predominance.
Panlobular Emphysema
Panlobular emphysema is characterized by uniform destruction of the pulmonary lobule, leading to widespread areas of abnormally low attenuation (Figs. 6-20 and 20-10 to 20-14, Table 20-2) (37,38,41,42). Thurlbeck (18) described this entity as a “diffuse ‘simplification’ of the lung structure with progressive loss of tissue until little remains but the supporting framework of vessels, septa and bronchi.” Involved lung appears abnormally lucent (41), a finding easy to appreciate in patients who have had unilateral lung transplantation (Figs. 20-11 and 20-13). Pulmonary vessels in the affected lung appear fewer and smaller than normal and may be quite inconspicuous. In contrast to centrilobular emphysema, panlobular emphysema almost always appears generalized or most severe in the lower lobes (Figs. 20-12 and 20-13).
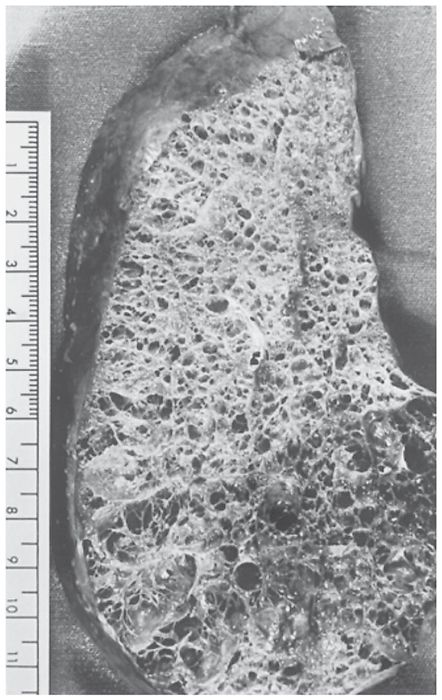
FIGURE 20-10 Panlobular emphysema. A pathologic specimen shows diffuse involvement of the parenchyma with simplification of lung architecture.
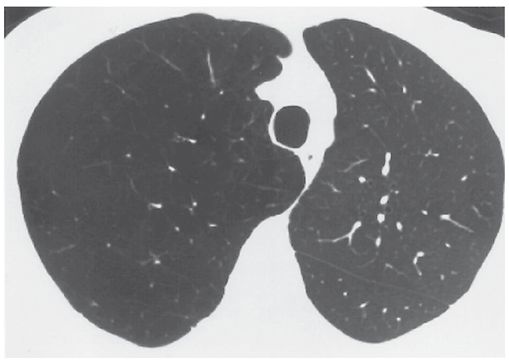
FIGURE 20-11 Panlobular emphysema in a patient who had left lung transplantation. The emphysematous right lung is relatively large and lucent and shows fewer and smaller vessels than are visible on the left. This appearance is typical of panlobular emphysema.
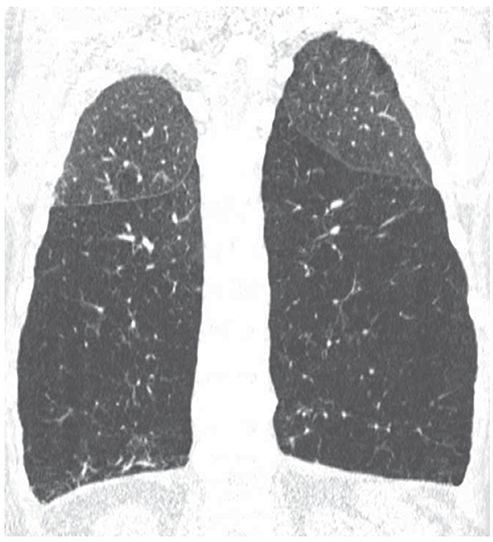
FIGURE 20-12 Panlobular emphysema due to alpha-1-antiprotease deficiency. Coronal HRCT reconstruction shows to good example the predominant lower lobe distribution of emphysema in patients with alpha-1-antitrypsin deficiency. There is marked simplification of lung architecture, with decreased vascular size, and the lower lobes are abnormally low in attenuation.
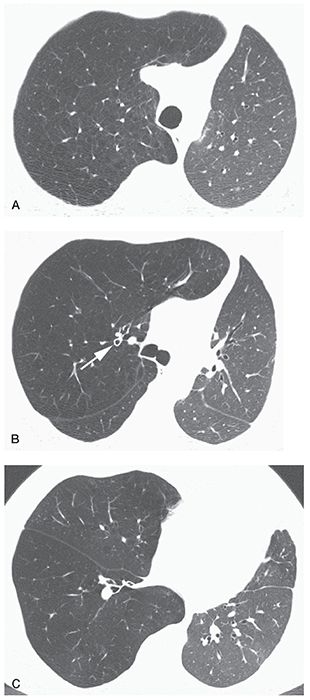
FIGURE 20-13 Panlobular emphysema associated with alpha-1-antitrypsin deficiency in a patient who had left lung transplantation. A: The emphysematous lung is lucent compared to the normal transplant and contains fewer and smaller vessels. Focal lucencies, seen in patients who have centrilobular or paraseptal emphysema, are absent in this patient, as are paraseptal emphysema and bullae. B: Findings of panlobular emphysema are also evident in the middle lung. Note bronchial wall thickening (arrow) as compared to the opposite side. The presence of bronchiectasis and bronchial wall thickening may be seen in patients who have alpha-1-antitrypsin deficiency. C: At the lung bases, findings of extensive emphysema are also visible. In panlobular emphysema, diffuse lung involvement is typical.
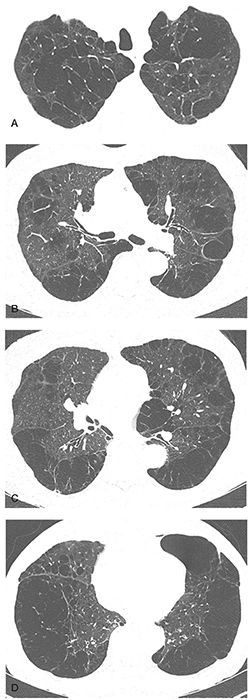
FIGURE 20-14 A–D: Panlobular emphysema associated with alpha-1-antitrypsin deficiency. Note that in this case both the upper and lower lobes are extensively involved with bullae more suggestive of centrilobular and paraseptal emphysema.
TABLE 20-2 HRCT Findings in Panlobular Emphysema
Lucent lung containing small pulmonary vesselsa,b |
Diffuse or lower lobe predominancea,b |
Focal lucencies and bullae less common than with centrilobular emphysemab |
Bronchiectasis or bronchial wall thickening |
aMost common findings.
bFindings most helpful in differential diagnosis.
Focal lucencies having the appearance of centrilobular emphysema are uncommon, but they may be seen in less abnormal lung regions (Fig. 20-14). Associated paraseptal emphysema and bullae are also uncommon, despite the severity of lung destruction. In one study (41), frank bullae were seen in 7 of 17 patients and were not considered a major feature of the disease.
In severe panlobular emphysema, the characteristic appearance of extensive lung destruction and the associated paucity of vascular markings are easily distinguished from normal lung parenchyma (Fig. 20-13). In contrast, mild and even moderately severe panlobular emphysema can be very subtle and difficult to detect (41). Furthermore, diffuse panlobular emphysema unassociated with focal areas of lung destruction or bullae may be difficult to distinguish from diffuse small airways obstruction and air trapping caused by bronchiolitis obliterans.
The diagnosis of panlobular emphysema is often associated with alpha-1-antitrypsin deficiency. It has been estimated that if screening was performed on the approximately 19 million white patients in the United States with emphysema, approximately 1.29 million patients with alpha-1-antitrypsin deficiency would be identified (43). In most patients with symptomatic alpha-1-antitrypsin deficiency, lung disease is the dominant clinical manifestation, usually presenting early in adulthood. Alpha-1-antitrypsin deficiency may be associated with bronchiectasis or bronchial wall thickening (Fig. 20-13B) (44). Presumably, patients who have alpha-1-antitrypsin deficiency are more susceptible to airways damage during episodes of infection than are normal patients because of the same protease-antiprotease imbalance that leads to emphysema. In a study by King et al. (45), 6 of 14 (43%) patients who had alpha-1-antitrypsin deficiency had CT evidence of bronchiectasis, a finding associated with symptoms of infection. Two patients had diffuse cystic bronchiectasis. Similarly, in a study by Guest and Hansell (41), bronchial wall thickening, dilatation, or both were present in 7 of 17 (41%) patients who had alpha-1-antitrypsin deficiency, with gross cystic bronchiectasis visible in 1 patient. Histologic findings in a patient who had alpha-1-antitrypsin deficiency and bronchiectasis visible on CT showed destruction of elastic lamina in ectatic bronchi and bronchioles (45).
The progression of panlobular emphysema associated with alpha-1-antitrypsin deficiency may be assessed using HRCT with densitometric measurements of lung attenuation (46,47) and has been found to be more sensitive than pulmonary function tests (PFTs) (47). In one study (47), 23 patients were scanned twice at a 1-year interval. HRCT was obtained at 90% of the vital capacity (VC), at the level of the carina, 5 cm above the carina, and 5 cm below the carina. During the follow-up period, mean lung densities decreased by 14.2 ± 12.0 HU above the carina, by 18.1 ± 14.4 HU at the carina, and by 23.6 ± 15.0 HU below the carina. In another study (46), 22 patients who had moderate emphysema associated with alpha-1-antitrypsin deficiency were followed for 2 to 4 years with an annual lung CT. A highly significant decline in CT-measured lung density was found in low-density areas, corresponding to a mean annual loss of lung tissue of 2.1 g/L lung volume.
Paraseptal Emphysema
Paraseptal emphysema is characterized by involvement of the distal part of the secondary lobule and is therefore most striking in a subpleural location (Figs. 6-23 to 6-25, 20-4 to 20-6, and 20-15 to 20-17). Areas of subpleural paraseptal emphysema often have visible walls, but these walls are very thin; they often correspond to interlobular septa. As with centrilobular emphysema, some fibrosis may be present. Even mild paraseptal emphysema is easily detected by HRCT (Table 20-3) (40).
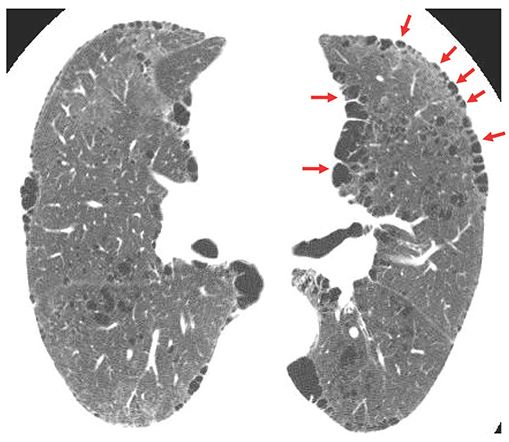
FIGURE 20-15 Paraseptal emphysema. HRCT shows focal subpleural lucencies (arrows) typical of paraseptal emphysema. These are commonly marginated by thin walls, which may correspond to interlobular septa. This patient also has centrilobular emphysema.
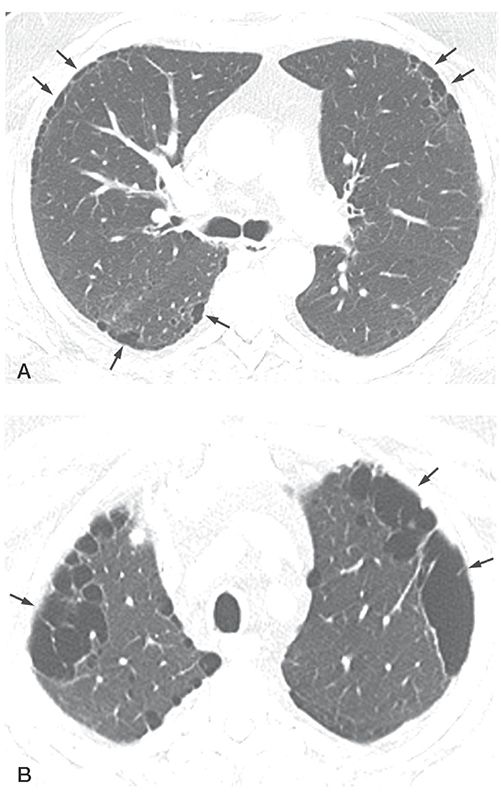
FIGURE 20-16 Paraseptal emphysema. A: HRCT at the level of the carina shows small, focal, subpleural lucencies (arrows) typical of paraseptal emphysema. These commonly have visible walls. B: HRCT at the level of the lung apices shows larger areas of subpleural paraseptal emphysema, termed bullae (arrows) because they are larger than 1 cm.
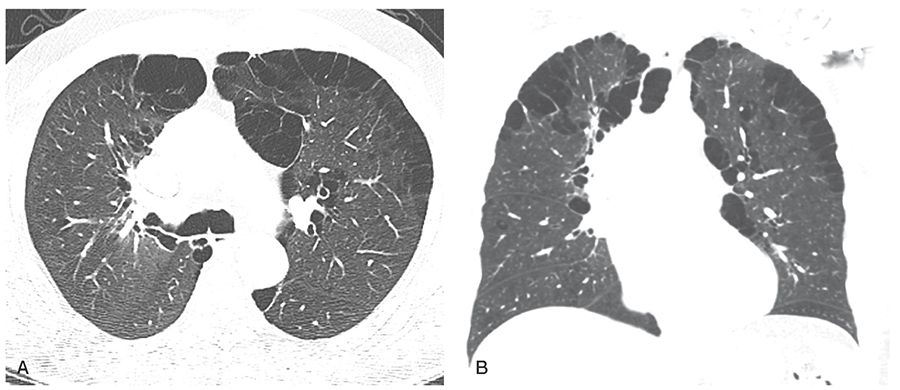
FIGURE 20-17 Paraseptal emphysema. A and B: HRCT through the carina shows the characteristic pattern of paraseptal emphysema with subpleural cysts. B: Coronal reconstruction shows an upper lobe predominance. Cysts larger than 1 cm are best termed “bullae.”
TABLE 20-3 HRCT Findings in Paraseptal Emphysema
Multiple, subpleural lucencies in a single layer, usually less than 1 cma,b |
Upper lobe predominancea,b |
Thin walls are commonly visiblea,b |
May be associated with centrilobular emphysema or bullae |
Pneumothorax may occur |
a most common findings.
b Findings most helpful in differential diagnosis.
When larger than 1 cm in diameter, areas of paraseptal emphysema are most appropriately termed bullae (Figs. 20-6 and 20-15 to 20-18). Subpleural bullae are frequently considered to be a manifestation of paraseptal emphysema, although they may be seen in all types of emphysema and as an isolated phenomenon. Regardless of the cause of the subpleural bullae, they usually have thin walls that are visible on HRCT.
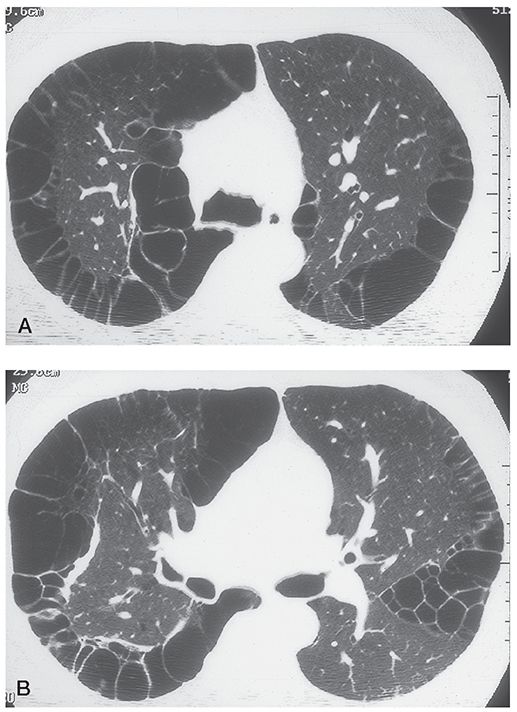
FIGURE 20-18 Paraseptal emphysema with large apical bullae. A and B: In a patient with paraseptal emphysema, numerous subpleural bullae are visible in the apices.
Lesur et al. (48) showed that CT may be useful in the early detection of apical subpleural bullae in patients who have idiopathic spontaneous pneumothorax (Fig. 20-19). This form of pneumothorax occurs most often in tall young adults (49) and is believed to be due to rupture of a subpleural bulla (48). Out of 20 patients (mean age, 27 ± 7 years), CT demonstrated emphysema in 17 patients with a predominance in the lung apices and in 16 patients with a predominance in a subpleural location.
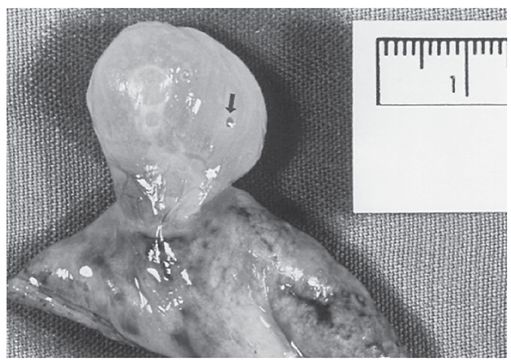
FIGURE 20-19 Paraseptal emphysema with a large subpleural bulla, which is exophytic in this resected specimen. This patient presented with pneumothorax. The small hole in the bulla wall (arrow) was presumably the site of air leak.
Bense et al. (50) also demonstrated that emphysema is seen on CT in the majority of nonsmoking patients who have spontaneous pneumothorax. They compared the CT findings in 27 nonsmoking patients who had spontaneous pneumothorax with the CT findings in 10 healthy subjects who had never smoked. Emphysema was present on CT in 22 of 27 nonsmoking patients who had spontaneous pneumothorax and in none of the 10 control subjects. The emphysema was present mainly in the periphery of the upper lung zones, a distribution consistent with paraseptal emphysema. In none of the cases was emphysema detected on a chest radiograph.
Similarly, in a prospective study of 35 patients who had primary spontaneous pneumothorax (51), the value of CT in detecting bullae was compared with that of chest radiographs. CT showed bullae or areas of subpleural emphysema in 31 of 35 (89%) patients, whereas chest radiographs showed these in only 11 (31%) patients. Six patients had a recurrent pneumothorax during follow-up; no correlation between recurrence and the number, size, and distribution of bullae as assessed by CT was found.
Bullous Emphysema
The term bullous emphysema does not represent a specific pathologic entity, but refers to the presence of emphysema associated with large bullae. It is generally seen in patients who have centrilobular emphysema, paraseptal emphysema, or both (Figs. 20-20 to 20-22) (21). A syndrome of bullous emphysema, or giant bullous emphysema, has been described on the basis of clinical and radiologic features and is also known as vanishing lung syndrome, type 1 bullous disease, or primary bullous disease of the lung (42). Giant bullous emphysema is often seen in young men and is characterized by the presence of large, progressive, upper lobe bullae, which occupy a significant volume of a hemithorax and are often asymmetric (Figs. 20-20 to 20-22). Arbitrarily, giant bullous emphysema is said to be present if bullae occupy at least one-third of a hemithorax (19). Most patients who have giant bullous emphysema are cigarette smokers (52), but this entity may also occur in nonsmokers.
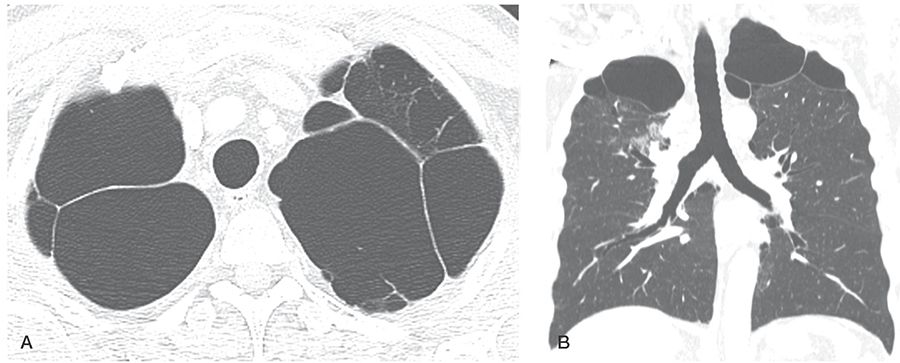
FIGURE 20-20 Bullous emphysema. A and B: Emphysema characterized primarily by bullae shows an upper lobe predominance.
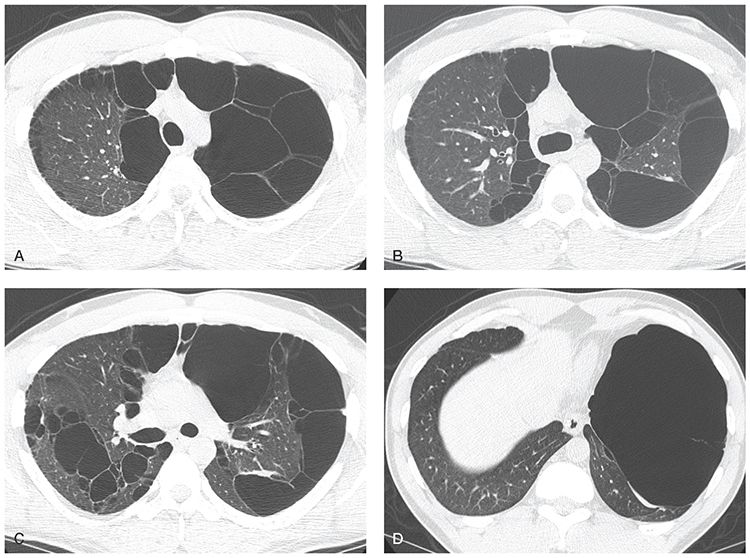
FIGURE 20-21 Bullous emphysema. A–D: HRCT shows large bullae, which predominate in the upper lobes and occupy most of the left lung. Asymmetry is common with bullous emphysema.
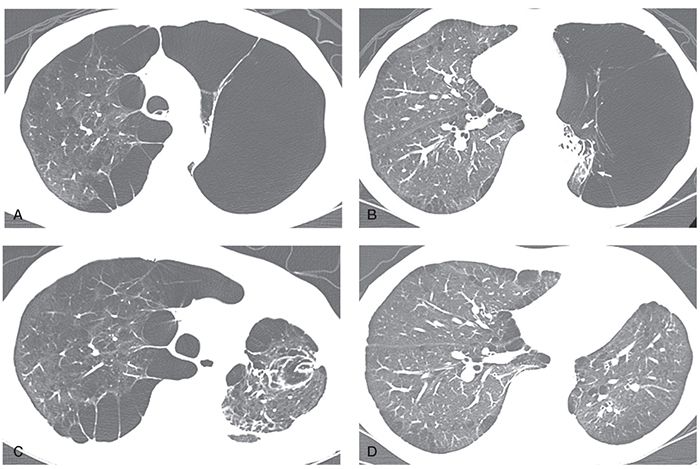
FIGURE 20-22 Giant bullous emphysema in a 50-year-old man. A and B: HRCT images show subpleural bullae associated with paraseptal and centrilobular emphysema in the right lung and giant bullous emphysema occupying a significant amount of the left hemithorax with collapse of the residual pulmonary parenchyma in the left lower lung (arrows in B). These findings are consistent with the vanishing lung syndrome. C and D: HRCT images performed after left bullectomy show re-expansion of the left lower lung. The patient improved symptomatically after surgery.
In nine patients who had giant bullous emphysema reported by Stern et al. (19), the most striking HRCT finding was the presence of multiple large bullae, varying in size from 1 to 20 cm in diameter, but usually between 2 and 8 cm in diameter (Figs. 20-21 and 20-22). Bullae were visible in a subpleural location and within the lung parenchyma, but subpleural bullae predominated. Bullae were often asymmetric, with one lung being involved to a greater degree. HRCT better depicted the presence of associated paraseptal and centrilobular emphysema than did chest radiographs. In this study (19), paraseptal emphysema was visible on HRCT in all subjects and was the predominant associated finding; centrilobular emphysema of varying degrees was present in eight of nine subjects.
Typically, bullae increase progressively in size. However, rarely, bullae may spontaneously decrease in size or disappear, usually as a result of secondary infection or obstruction of the proximal airways (53). Spontaneous pneumothorax is common and may be recognized only on CT (48,51). So-called “infected bullae” are also common, typically resulting from fluid accumulation secondary to the presence of adjacent foci of parenchymal infection (Fig. 20-23). Despite being labeled as “infected,” these fluid collections are typically sterile, analogous to simple transudative pleural effusions that occur in patients with an underlying pneumonia. It should be emphasized that these fluid collections may persist for extended periods of time, frequently months before resolving, and that consequently they should be followed conservatively in the absence of complications. Fluid may also accumulate within a bulla because of hemorrhage or neoplasm.
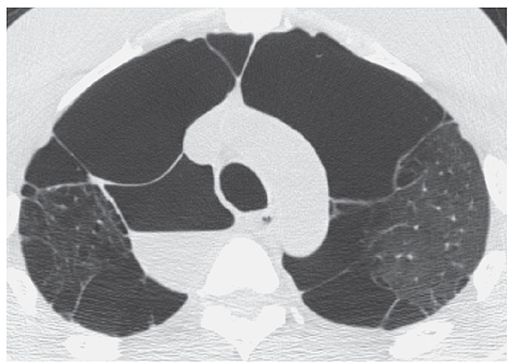
FIGURE 20-23 “Infected bulla.” HRCT through the lung apices shows a thin-walled paramediastinal bulla, containing an air-fluid level, consistent with the diagnosis of an “infected bulla.” Usually the result of prior infection in the adjacent lung, these fluid collections are generally sterile, similar to the transudative effusions that occur secondary to underlying pneumonia, and may persist for months.
Irregular Airspace Enlargement
Irregular airspace enlargement, previously known as irregular or cicatricial emphysema, is commonly found adjacent to localized parenchymal scars, diffuse pulmonary fibrosis, and in the pneumoconioses, particularly progressive massive fibrosis (54). It is most easily recognized on CT when the associated fibrosis is identified. However, this type of emphysema may also be seen associated with microscopic fibrosis, in which case radiologic distinction between irregular and centrilobular emphysema may be impossible (55).
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
The Global Initiative for Chronic Obstructive Pulmonary (Lung) Disease (GOLD) (56) defines COPD in functional terms, as persistent, usually progressive airflow limitation established spirometrically, as a decrease in the FEV1/FVC to less than 0.70, that cannot be reversed following bronchodilator therapy.
COPD is currently the 12th leading cause of disability in the world and is predicted to be the 5th by 2020 (57). As reported by the Centers for Disease Control and Prevention, a total of 3.8 million noninstitutionalized adults suffered from COPD in 2008 (58). Respiratory symptoms in patients who have COPD are nonspecific and include chronic cough, sputum production, and dyspnea, among others. Although cough and sputum production have traditionally been considered manifestations of chronic bronchitis, the relative contributions of airways disease and emphysema to respiratory disability are often difficult to determine.
Four stages of COPD severity have been arbitrarily defined (GOLD I–IV) with which an individual’s values can be compared. Practical in application, this approach is limited by providing only a global assessment of disease and is insensitive to asymptomatic patients and those with mild symptoms. In fact there is now a consensus that COPD represents a wide spectrum of phenotypes which, by definition, broadly include physiologic, clinical, and radiologic patterns of overlapping diseases manifesting considerable variability (59). COPD is a complex disorder involving both pulmonary and extrapulmonary components, in which genetic factors interact with environmental antigens, especially tobacco smoke, resulting in an exaggerated inflammatory response that ultimately destroys lung parenchyma, resulting in a loss of elastic recoil (emphysema), and/or remodels the peripheral airways due to mucous gland hyperplasia, bronchoconstriction, and airway narrowing (chronic bronchitis and/or small airway disease) (60).
Morphologic differences among patients with airway obstruction reflect important differences in the underlying pathophysiology and genomic profiles of patients diagnosed with COPD (61,62). CT clearly demonstrates that the degree of airway obstruction correlates poorly with the anatomical extent of emphysema, especially when it is predominantly upper lobe and centrilobular in distribution, with r values ranging from 0.40 to 0.70 (22,24,25). CT evidence of emphysema is frequently found in patients who do not meet spirometric criteria for COPD (63,64), while in distinction, emphysema can involve 30% of the lung parenchyma without airflow obstructions being present (22,24,25). In addition to limitations on the use of a single physiologic measure of airway obstruction to define clinical, physiologic, and radiologic subtypes, reliance on FEV1 has also proved of limited prognostic value. As a consequence, numerous additional phenotypes have been proposed reflecting the diversity of clinical, physiologic, and radiologic manifestations of COPD (56,65).
Phenotypes in COPD
As proposed by Han and colleagues (66), COPD phenotyping can be defined “as a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbation, response to therapy, rate of disease progression, or death).”
Examples of proposed clinical phenotypes include patients with dyspnea, frequent exacerbations, impaired exercise tolerance, and pulmonary cachexia, and nonsmokers, among others. Proposed physiologic phenotypes include rapid decliners, airway hyper-responsiveness, hypercapnia, low carbon monoxide diffusing capacity (DLCO), and pulmonary hypertension, among others (59).
CT can serve to complement clinical and physiologic phenotypes and may potentially provide insight into underlying genetic patterns of disease (66–71) (Table 20-4). Presently, two dominant CT phenotypes have been proposed: emphysema-predominant disease, and airway-predominant disease resulting from chronic airway inflammation with resulting airway remodeling and narrowing.
TABLE 20-4 Visually Defined Patterns of COPD on CT

Emphysema-Predominant Disease
Given that HRCT is well established as a method of assessing the presence, pattern, and severity of both microscopic and macroscopic emphysema, identifying a pattern of emphysema-predominant COPD is generally straightforward, especially for patients with higher GOLD grades (72–80).
Emphysema-predominant disease (Fig. 20-24, Table 20-4) can be subdivided according to the type of emphysema present (centrilobular, panlobular, paraseptal, and/or bullous emphysema) and further characterized by the anatomical distribution and severity of abnormalities using either visual or quantitative computed tomography (QCT) techniques. Although centrilobular and panlobular emphysema are most commonly encountered in patients with emphysema-predominant disease, only 44% to 59% exhibit pure patterns of disease (59,81–83). In addition to considerable overlap, differentiation between severe centrilobular and panlobular emphysema may prove problematic, as is the frequent presence of associated paraseptal emphysema.
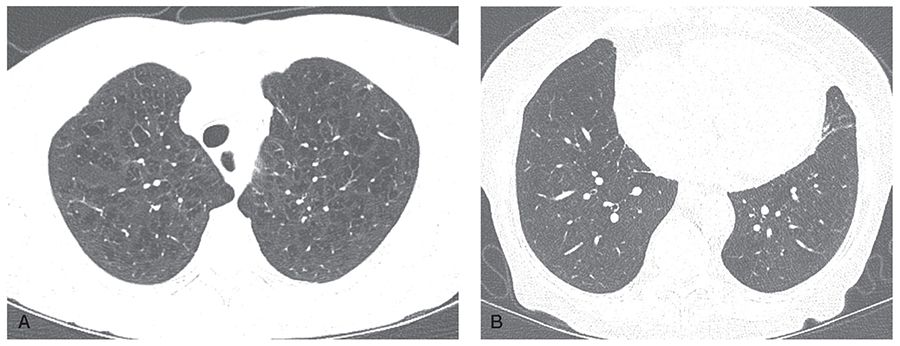
FIGURE 20-24 COPD: emphysema-predominant phenotype. A: HRCT section through the upper lobe in a patient with GOLD stage III disease shows moderate to severe upper lobe centrilobular emphysema. B: HRCT section through the proximal lower lobe airways shows airways to appear normal in size and wall thickness. This constellation of findings is characteristic of emphysema-predominant COPD.
Airway-Predominant Disease
In distinction to emphysema-predominant COPD, airway-predominant COPD is defined by a relatively minor degree of emphysema associated with marked airway wall abnormalities, in particular, airway wall thickening (Fig. 20-25, Table 20-4). Critical to our understanding of the potential role of CT to define a specific airway-predominant phenotype among patients with COPD are two seminal papers documenting that evaluation of larger airways may serve as an indirect measure of small (<2-mm-sized) airway abnormalities. As shown by Nakano et al. (84), CT measurements of airway wall area coupled with measurements of luminal dimensions can be used to differentiate patients with asthma (who exhibit increased airway wall area but normal luminal dimensions) from patients with COPD (who exhibit both increased wall area and decreased luminal dimensions). Critically, in this study, correlation was established between increased airway wall area as measured in larger airways and anatomical evidence of increased airway wall area in small airways (84).
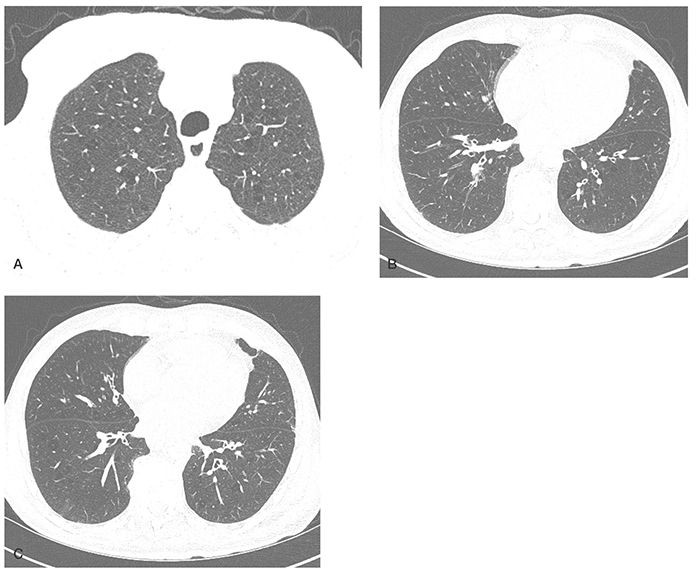
FIGURE 20-25 COPD: airway-predominant phenotype. A: HRCT section through the upper lobe in a patient with GOLD stage III disease shows only mild centrilobular emphysema. B and C: Sections though the proximal lower lobe airways show evidence of diffuse bronchial wall thickening. This constellation of findings is characteristic of airway-predominant COPD.
In a follow-up study that evaluated 22 smokers who underwent pneumonectomy or lobectomy for resection of small peripheral lung nodules, Nakano et al. (85) evaluated whether the dimensions of relatively large airways assessed by CT reflected small airways dimensions measured histologically. The authors found that CT measurements of airways with an internal perimeter larger than 7.5 mm on CT (corresponding to an internal diameter larger than 2.4 mm) reflect the dimensions of small airways measured at pathologic examination, and therefore could be used to estimate the dimensions of the small conducting airways, which are the site of airway obstruction in COPD (85).
The pathologic abnormalities in the small airways of patients with COPD that result in airflow obstruction include luminal occlusion by inflammatory mucous exudates, luminal encroaching by epithelial layer thickening, airways smooth cell hypertrophy and contraction, deposition of connective tissue in the adventitial compartment of the airway wall, destruction of the support from alveolar attachments, and mucus secretion that alters airway surface tension and predisposes to expiratory collapse (86,87). Both innate and adaptive inflammation including neutrophils, macrophages, CD4+ cells, CD8+ cells, and B cells contribute to bronchial wall thickening, with disease progression also associated with an increase in the number of peribronchial lymphoid follicles (61). In addition, these changes are secondarily associated with loss of elastic recoil reducing airway dilatation on inspiration (88–90). Further documentation that airway wall thickness is related to symptoms of chronic bronchitis, including chronic cough, and wheezing, not related to emphysema extent, has been reported. In fact, QCT may be used to explain the presence of respiratory symptoms beyond the information offered by spirometry. Grydeland et al. (91) evaluated the correlation of respiratory symptoms with measurements of emphysema and airway wall thickness in 463 patients with COPD. They found that the percentage of lung parenchyma with low-attenuation (less than –950 HU) areas and the mean standardized airway wall thickness at an internal perimeter of 10 mm were independently related to dyspnea in subjects with COPD, even when adjusting for percent predicted FEV1. Airway wall thickness at an internal perimeter of 10 mm was significantly related to morning cough, chronic cough, and wheezing attacks in subjects with COPD and to wheezing attacks in subjects without COPD (91).
Small Airways Disease in COPD
Although small airway disease is present in both emphysema and airway-predominant COPD, it may also be seen as a primary manifestation of COPD in the absence of HRCT findings of emphysema or large airway disease (92). Clinically, small airway disease may be suspected in the setting of a low FEV1 and FEV1/FVC ratio, although this remains an insensitive method of diagnosis. More reliable is the finding of air trapping on paired inspiratory/expiratory CT studies. Normally, on expiration, overall lung volumes diminish and lung density increases consistent with a decreased volume of air within the lungs. In smokers, expiratory scans may show either diffuse or, more frequently, localized regions of air trapping, identifiable as areas of relatively low attenuation when compared with normal lung. This is attributable to focal constrictive bronchiolitis, and, in part, a loss of adjacent alveolar attachments. Not infrequently, these changes are accompanied by a decrease in the size of accompanying pulmonary vessels resulting in so-called mosaic attenuation or mosaic perfusion.
The utility of paired inspiratory and expiratory HRCT for the quantitation of air trapping in patients with COPD, regardless of the presence of underlying emphysema, has been reported by several authors. For example, Matsuoka et al. (93) in a study of 36 patients with COPD determined the extent of emphysema on inspiratory scans based on the relative lung volume with an attenuation of less than –950 HU. By subsequently limiting whole lung segmentation to areas with an inspiratory attenuation of between –500 and –950 HU, these authors excluded areas of emphysema, and were able to obtain a measurement of the extent of air trapping in nonemphysematous lung. The use of expiratory HRCT is discussed further in what follows and also in Chapter 7.
Less commonly, small airway disease presents with ground-glass opacity centrilobular nodules, typically measuring a few millimeters in size, and characteristically predominating in the upper lobes. These findings almost always correlate with the presence of mild respiratory bronchiolitis and in themselves are indicative only of a smoking history. When accompanied by ill-defined areas of ground-glass opacity, in addition to small airway-predominant COPD, differential diagnosis includes respiratory bronchiolitis-interstitial lung disease or less likely desquamative interstitial pneumonitis.
CT Imaging Techniques for Evaluating Patients with COPD
Our ability to diagnose and quantitate the extent and severity of both emphysema and airway abnormalities by CT is influenced by a number of technical factors. These include collimation, window settings, the threshold values chosen for diagnosing emphysema, radiation dose, reconstruction algorithm, phase of respiration, use of intravenous contrast media, volumetric versus axial reconstruction, and differences among individual scanners (Table 20-5) (72,73,94–102). Additional factors to be considered include use of expiratory and/or paired inspiratory-expiratory images, as well as the use of spirometric gating (78,103–106).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


