Hypertrophic Cardiomyopathy: Introduction
Hypertrophic cardiomyopathy (HCM) is usually an autosomal dominant disease of the heart muscle, with an overall prevalence of 1:500 to 1:1000, characterized by a small left ventricular cavity and marked hypertrophy of the myocardium with myofibril disarray.1-8
The basis of HCM has been ascribed to multiple etiologies9,10; however, in 1989, investigators first mapped a genetic mutation for HCM to chromosome 14.11,12 This mutation later was shown to effect the encoding of β-myosin heavy chain protein, which is a major component of the cardiac sarcomere.13,14 Subsequently, hundreds of mutations have been found in HCM patients; most of these mutations involve the myofilaments of the cardiac sarcomere; however, there is increasing awareness of nonsarcomeric mutations as well.15,16 Thus, HCM, in its most common manifestation, is felt to be a disease of the myofilaments, whose alteration in structure and/or function underlies the pathology and pathophysiology in affected individuals.17-27
HCM is a highly heterogeneous disease, with a diverse pathology and clinical course.4-7 Some patients may present with severe limiting symptoms of dyspnea, angina, and syncope, and yet other patients may remain completely asymptomatic throughout life. HCM is the most common cause of sudden cardiac death in young people, including trained athletes.28-34 Yet, the overall prognosis of HCM patients is comparable to that of an age-matched, sex-matched control population.4,35-41
Historical Perspective
The first anatomic description of HCM was by Teare in 1958,42 when he reported the pathologic findings in eight young patients, seven of whom had died suddenly. He found massive hypertrophy of the ventricular septum with microscopic evidence of myocardial disarray of the individual muscle fibers, which was speculated to be due to either a benign tumor or hamartoma. At the same time, Brock43 reported on patients with functional subvalvular left ventricular outflow tract gradients confirmed by pressure gradients. Braunwald and colleagues, in the 1960s, defined the specific disease process, in which asymmetric septal hypertrophy, myofibril disarray, and a dynamic subvalvular pressure gradient were documented.44,45 This entity was considered to be a primary process with no known cause; however, a genetic connection was suggested when a familial link was shown to be associated with this entity in an autosomal dominant fashion.46
Since these initial descriptions, the disease process has come to be known by many names, including asymmetric septal hypertrophy, idiopathic hypertrophic subaortic stenosis, muscle subaortic stenosis, and hypertrophic obstructive cardiomyopathy. The World Health Organization has designated the term hypertrophic cardiomyopathy (HCM) to describe this unique process of primary muscle hypertrophy, which may exist with or without a dynamic left ventricular outflow tract gradient.47,48
The evolution of echocardiography provided a noninvasive diagnostic tool to identify patients with HCM.49-51 Echocardiography showed that hypertrophy in HCM patients could involve areas other than the ventricular septum and further led to the understanding of the complex pathophysiology of HCM.1,52 It is now recognized that the severity and distribution of hypertrophy may be highly variable and that there are some patients with HCM who may have only mild hypertrophy yet still be at risk for disease complications such as sudden death.33,53,54
Early descriptions of HCM patients, which originated from several large referral centers, suggested that there were high mortality rates (3%-7% annually) including many sudden deaths.3-5,55-57 These studies were comprised of selected younger patients who had been referred because they had been judged to have either a high-risk status or severe symptoms requiring highly specialized care. More recent reports from nontertiary centers with less selected, regional, or community-based cohorts have revealed that the overall mortality of patients with HCM is similar to that of control populations.4,35-41 It is now well accepted that although there still remains a select group of patients who are identified as being at high risk for sudden catastrophic events, the majority of HCM patients live an uneventful, asymptomatic life.4
Etiology
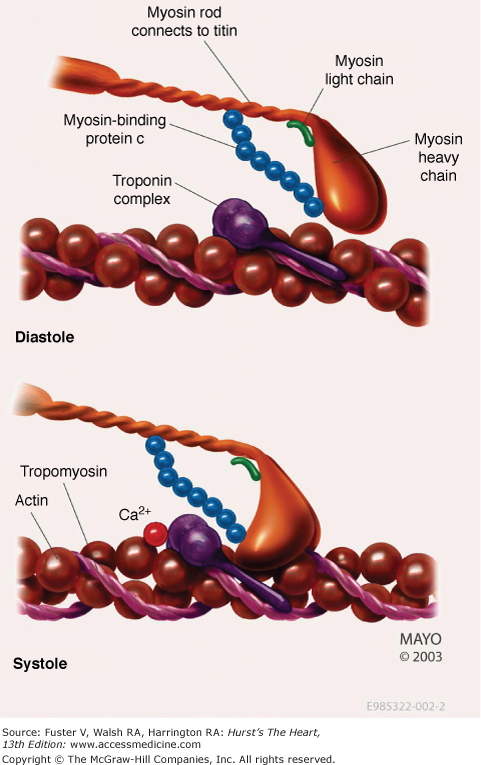
Investigators now believe that HCM is the most common genetic cardiovascular disease. Hundreds of mutations have been associated with clinical HCM with the vast majority encoding proteins of the cardiac sarcomere myofilaments (Fig. 33–1).17-20,24,27 Affected genes include β-myosin heavy chain, myosin-binding protein C, cardiac troponin T and I, α-tropomyosin, actin, titin, and myosin light chains.53,54,58-71 These known mutations are inherited in an autosomal dominant manner. Importantly, mutations in other genes outside of the sarcomeric myofilaments have also been implicated in HCM.72 A genetic locus has been mapped to chromosome 7 in patients with cardiac hypertrophy and electrophysiologic abnormalities such as Wolff-Parkinson-White syndrome.73-75 This gene encodes the y2 regulatory subunit (PRKAG2) of adenosine monophosphate–activated protein kinase, an enzyme that modulates glucose metabolism. Histopathologic and biochemical studies of the myocardium have shown myocyte enlargement with glycogen-filled vacuoles, suggesting that these patients who in the past were diagnosed as having HCM may have a different disease process similar to a glycogen storage disease.
Several types of mutations have been identified in these genes, including deletions, insertions, and missense and splice site mutations.26,27 Mutant peptides that are encoded affect function of the contractile unit of the myofilaments by becoming incorporated into the sarcomere itself.17,18,76 The different mutations may result in a different biophysical consequence. Some defects may alter the actin-myosin crossbridge formation, and others may affect the movement and force generation of the thick and thin filaments. It has been hypothesized that the sarcomeric dysfunction can lead to “compensatory” myocardial hypertrophy, although the precise impetus for hypertrophy has not yet been identified.17,18,76-78 As evidenced by the large number of both sarcomeric- and nonsarcomeric-related mutations and the lack of a clearly identified final common pathway, there appears to be an important role for other genetic and/or environmental modifiers.79-82 Ultimately, there may be a large number of genes that influence the phenotypic expression of HCM. Although the role of specific mutations is not uniformly agreed upon, the specific morphologic features of the hypertrophy appear to be different among those patients whose disease is based on the presence of mutations in the sarcomeric myofilaments compared with those who do not harbor such a mutation.83
Pathology
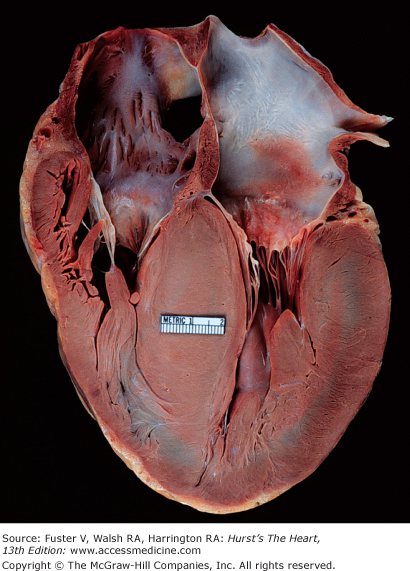
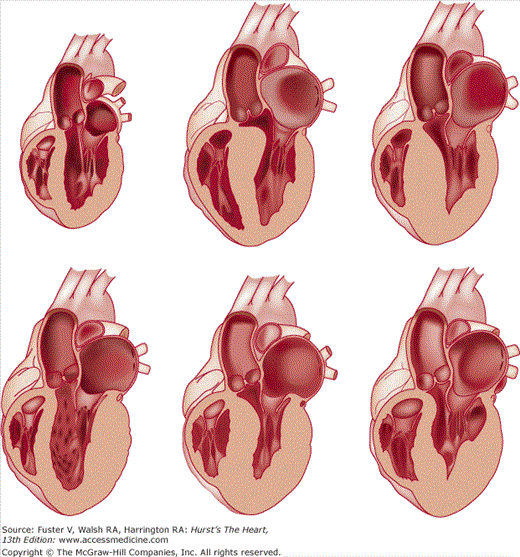
Gross examination of the heart in HCM demonstrates asymmetric septal hypertrophy with a small left ventricular cavity (Fig. 33–2).84-86 The mural endocardium may be thickened by fibrous tissue, and if left ventricular outflow tract obstruction is present, there is often a plaque located on the upper septal area where the mitral valve repeatedly has come in contact with the septum during systole. The mitral valve itself may be intrinsically normal; however, there may be elongation of the mitral chordae and anterior displacement of hypertrophied papillary muscles. Abnormal attachments of the mitral valve chordal apparatus to the septum and attachments of the papillary muscle head directly into the leaflets may be found. The left atrium is usually dilated at autopsy. Although the epicardial coronary arteries are normal, the intramural coronary arterioles in the septum are small because of intimal hyperplasia and increased in number.87,88
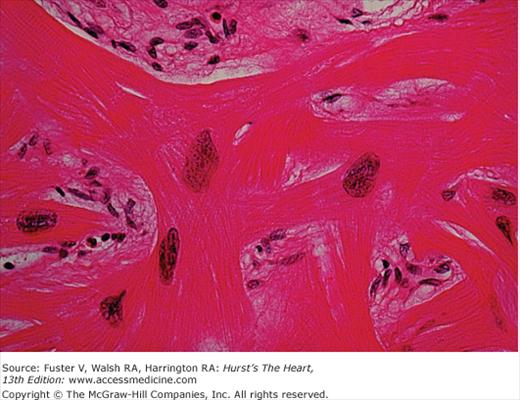
In the original report by Teare42 bizarre arrangements of the muscle fiber bundles separated by clefts lined with endothelin were described. The myocardial disarray consists of short runs of severely hypertrophied fibers interrupted by connective tissue. There are large and bizarre nuclei and fibrosis present, with degenerating muscle fibers. This disorganization results in a “whorling” of muscle fibers that is characteristic of HCM (Fig. 33–3). Disarray can be observed throughout the myocardium, is not specific to HCM, and can be seen in any pressure-overloaded ventricle; although, the proportion of myocardial disarray is much greater in HCM patients.89,90
Pathophysiology
The pathophysiology of HCM is complex and consists of multiple interrelated abnormalities, including diastolic dysfunction, left ventricular outflow tract obstruction, mitral regurgitation, myocardial ischemia, and arrhythmias.1,2,4,6,7
A major pathophysiologic abnormality in HCM is diastolic dysfunction arising from multiple factors, which ultimately affect both ventricular relaxation and chamber stiffness (Fig. 33–4).1,2,91-93 An impairment of ventricular relaxation results from the high systolic contraction load caused by an outflow tract obstruction, nonuniformity of ventricular contraction and relaxation, and delayed inactivation caused by abnormal intracellular calcium reuptake. The severe hypertrophy of the myocardium results in an increase in chamber stiffness. Diffuse myocardial ischemia may further affect both relaxation and chamber stiffness. Associated with these alterations is a compensatory increase in the contribution of late diastolic filling during atrial systole. With exercise or any other type of catecholamine stimulation, the decrease in diastolic filling period and myocardial ischemia will further lead to severe abnormalities of diastolic filling of the heart, with an increase in pulmonary venous pressure causing symptoms of dyspnea.
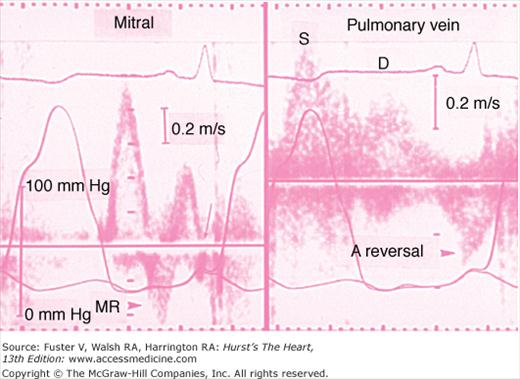
FIGURE 33–4
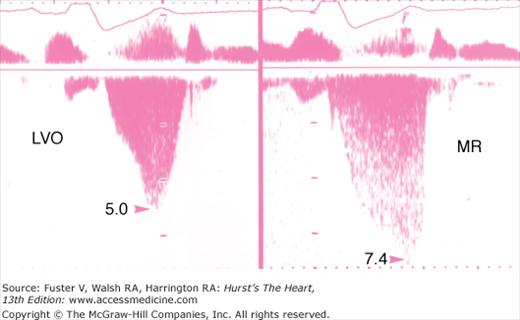
Evidence of severe diastolic dysfunction in a patient with hypertrophic cardiomyopathy. The left ventricular and left atrial pressures are shown in both the left and right panel. The mean left atrial pressure is severely elevated to 30 mm Hg. Left: The mitral flow velocity curve is shown, demonstrating a high E:A ratio and a short deceleration time. Diastolic mitral regurgitation (MR) is present. There is abrupt cessation of the “a” duration (arrow). Right: The pulmonary vein velocity curve is shown, with a high velocity at atrial reversal of long duration. The systolic forward flow (S) and diastolic forward flow (D) are shown.
The original observations by Brock and Braunwald emphasized the functional subvalvular left ventricular outflow tract gradient, which was highly influenced by alterations in the load and contractility of the left ventricle (Fig. 33–5).43,44 The clinical significance of the outflow tract gradient has been controversial, but careful studies have shown definitively that true obstruction does exist.1,2,94-96 The obstruction causes an increase in left ventricular systolic pressure, which leads to a complex interplay of abnormalities including prolongation of ventricular relaxation, elevation of left ventricular diastolic pressure, mitral regurgitation, myocardial ischemia, and a decrease in forward cardiac output.1,2
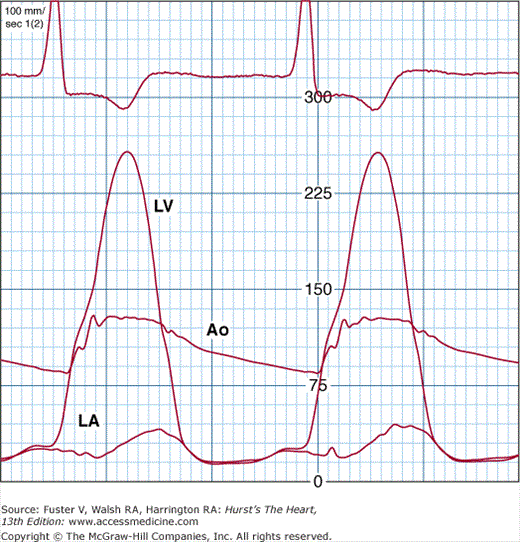
FIGURE 33–5
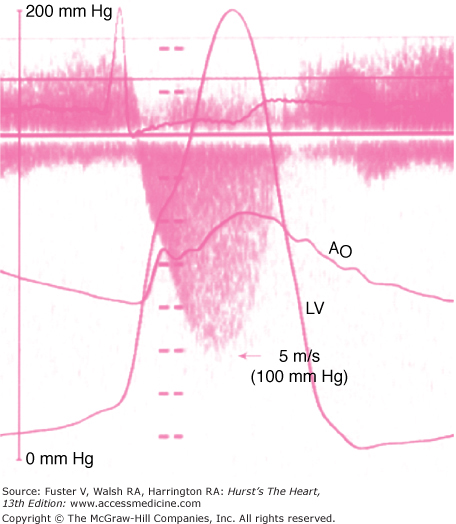
Cardiac catheterization pressure curves showing a severe left ventricular outflow tract obstruction. The gradient between the left ventricle (LV) and aorta (Ao) is nearly 100 mm Hg. The left atrial (LA) pressure is also elevated. There is a “spike and dome” pattern on the aortic pressure curve.
The mechanism of obstruction is multifactorial. The obstruction was initially thought to be due to systolic contraction of the hypertrophied basal ventricular septum, which would then encroach into the left ventricular outflow tract with a resultant suction, or Venturi force, that would pull the mitral valve leaflets into the left ventricular outflow tract and produce further obstruction.1,2,97 Elegant studies have emphasized that during ventricular systole, flow against the abnormally positioned mitral valve apparatus will result in a drag force on a portion of the mitral valve leaflets and actually “push” the leaflets into the outflow tract.98-101 Obstruction can also be present in the midcavitary region due to hypertrophied papillary muscles abutting against the septum.102
The obstruction to left ventricular outflow is dynamic, varying with loading conditions and contractility of the ventricle (Fig. 33–6).44,103 An increase in myocardial contractility, a decrease in ventricular volume, or a decrease in afterload will increase the degree of subaortic obstruction. Patients may have little or no obstruction to left ventricular outflow at rest but can generate large left ventricular outflow tract gradients under conditions such as exercise, the strain phase of the Valsalva maneuver, or pharmacologic provocation. It has been well established that this dynamic left ventricular outflow tract obstruction contributes in part to the debilitating symptoms that may occur in these patients and is an important determinant of outcome in HCM.104
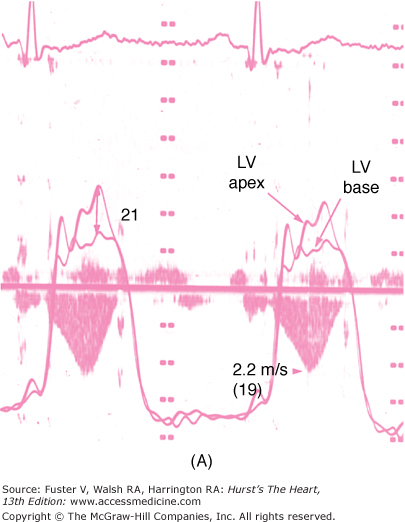
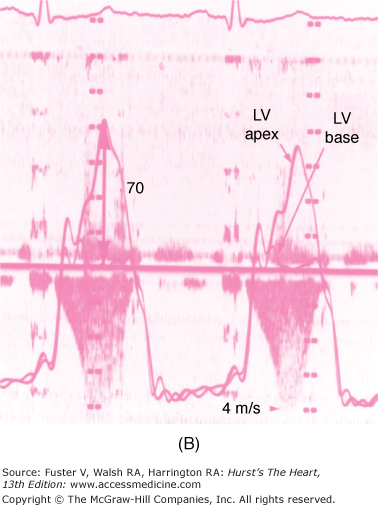
FIGURE 33–6
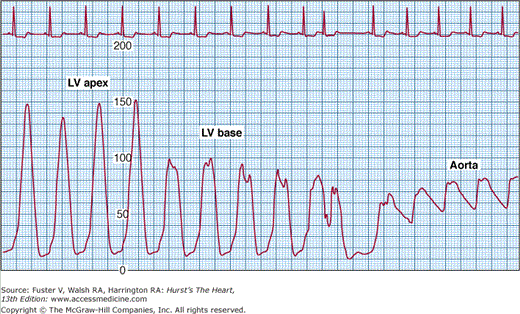
The dynamic nature of the left ventricular outflow tract obstruction is shown by simultaneous Doppler echocardiography and cardiac catheterization. The cardiac catheterization is performed with a pressure measurement of the left ventricular apex and left ventricular base. The gradient occurs during systole between the left ventricular apex and left ventricular base. The continuous wave Doppler velocities through the left ventricular outflow tract are shown. The calculated gradient from the Doppler echocardiogram is shown in parentheses. A. The gradient in the baseline state is 21 mm Hg as assessed by both cardiac catheterization and Doppler echocardiography. B. During inhalation of amyl nitrite, the gradient increases to 70 mm Hg.
Severe myocardial ischemia and even infarction may occur in HCM.87,105,106 The myocardial ischemia is frequently unrelated to the atherosclerotic epicardial coronary artery disease, but rather due to supply-demand mismatch. Patients with HCM have increased oxygen demand due to the hypertrophy and adverse loading conditions but also have compromised coronary blood flow to the left ventricular myocardium because of abnormally small and partially obliterated intramural coronary arteries.87,88,105,107
During exercise, approximately 25% of HCM patients will have an abnormal blood pressure response defined by either a failure of systolic blood pressure to increase >20 mm Hg or a decrease in systolic blood pressure.108,109 The presence of this finding is associated with a poorer prognosis.109,110 This inability to augment and sustain systolic blood pressure occurs despite an appropriate increase in cardiac output and may be due to systemic vasodilatation during exercise. It is speculated that there is a high degree of abnormal autonomic tone in HCM. A contribution from exercise-induced augmentation of outflow tract obstruction must also be considered.
Mitral regurgitation is common in patients with left ventricular outflow tract obstruction and may play a primary role in producing symptoms of dyspnea.1,2,111 The temporal sequence of events of eject-obstruct-leak supports the concept that the mitral regurgitation in most patients is a secondary phenomenon.1,2,111 The mitral regurgitation is usually caused by the distortion of the mitral valve apparatus from the systolic anterior motion secondary to the left ventricular outflow tract obstruction. The jet of mitral regurgitation is directed laterally and posteriorly and predominates during mid and late systole (see Fig. 33–6), and the severity is proportional to the left ventricular outflow tract obstruction. Changes in ventricular load and contractility that affect the severity of outflow tract obstruction will similarly affect the degree of mitral regurgitation. It is important to identify patients who have additional intrinsic disease of the mitral valve apparatus (prolapse or flail) because this finding will influence subsequent treatment options.112
Clinical Presentation
There is a wide variability in the clinical presentation of HCM patients.3,4 The majority of patients who have HCM are asymptomatic, with the disease being diagnosed on the basis of abnormal electrocardiogram, heart murmur, or screening echocardiogram. When symptoms are present, dyspnea, angina, and/or syncope are the most common. The symptoms often vary from day to day in individual patients. Additionally, sudden death may occur, particularly in the young population.
Dyspnea occurs in up to 90% of symptomatic HCM patients. The stiff, noncompliant, hypertrophied ventricle and abnormal relaxation cause elevated left ventricular filling pressures, a situation that is exacerbated with exercise. The diastolic filling abnormalities are augmented by the occurrence of a dynamic left ventricular outflow tract obstruction or concomitant mitral regurgitation.1,2 However, dyspnea may occur in the absence of outflow tract obstruction or mitral regurgitation, if there is severe diastolic dysfunction.
Angina occurs in 70% to 80% of patients with symptomatic HCM, and 15% of all patients at autopsy have associated myocardial infarction.87 The angina may frequently occur in the absence of epicardial coronary disease, related to a number of mechanisms including small artery narrowing, intramural compression of small arteries from myocardial hypertrophy, abnormal diastolic filling, oxygen supply-demand mismatch, and abnormal coronary flow reserve.
Syncope and presyncope occur in approximately 20% and >50% of HCM patients, respectively. Syncope can be due to either a hemodynamic or rhythm abnormality. It has been speculated that activation of left ventricular baroreceptors results in a reflex vasodilatation, and that mechanism may be worsened by a dynamic left ventricular outflow tract gradient. The syncope in this case occurs either during or immediately after exercise.
Patients with HCM often describe an increase in symptoms during hot humid weather, presumably due to fluid loss and vasodilation resulting in decreases in both preload and afterload. Similarly, symptoms also may be more prominent after eating a large meal or after drinking alcohol. Other concomitant problems such as anemia or fever may exacerbate symptoms.
Older patients may present differently than younger patients.4,5,113-116 Many of these patients will have had a history of hypertension and will thus present on medications such as diuretics and vasodilators, which exacerbate outflow tract obstruction. Atrial fibrillation occurs commonly in older patients (up to 25%-30%) and may herald systemic embolism and further clinical deterioration.4,35,39 These older patients may also have manifestations of other concomitant cardiac disease, such as coronary artery disease, degenerative valvular aortic stenosis, and mitral annular calcification.
Physical Examination
The classic physical findings of HCM apply to patients who have a left ventricular outflow tract gradient. The HCM patients who do not have an outflow tract obstruction should have findings of left ventricular hypertrophy on physical examination.
The classic carotid pulsation is brisk with a spike and dome pattern, characterized by a rapid rise (percussion wave) followed by a midsystolic drop that is, in turn, followed by a secondary wave (tidal wave). The midsystolic drop in amplitude of the carotid pulse contour is caused by premature closure of the aortic valve and coincides with systolic anterior motion of the mitral valve. The late peak is due to relief of the outflow tract gradient as the mitral valve leaflet returns to its original position. In the presence of pronounced obstruction, there is a longer ejection time. The carotid pulsation with dynamic left ventricular outflow tract obstruction differs from that of a fixed obstruction, such as seen with valvular or discrete subvalvular aortic stenosis where there is a decrease in both the rate of rise and the amplitude of the pulsation.
The jugular venous pressure is normal in most HCM patients. However, the a wave may be prominent, indicating a decrease in compliance ventricle due to the hypertrophy of the right ventricular free wall or septum, pulmonary hypertension from left-sided diastolic pressure elevation, or right ventricular outflow obstruction.
The apical impulse is almost always abnormal in patients with HCM and reflects the myocardial hypertrophy. The apical impulse is a sustained systolic thrust that continues throughout most of systole. There is frequently a bifid impulse due to a forceful atrial systole. There may be a triple ripple, with a third component occurring near the end of systole if outflow tract obstruction is present. A systolic thrill may be palpable at apex from severe mitral regurgitation or at the lower left sternal border from outflow tract obstruction.
Auscultation usually reveals a normal or loud first heart sound. The second heart sound is usually split physiologically, although approximately 20% of patients may have a paradoxical split due to either a concomitant left bundle-branch block or severe left ventricular outflow tract obstruction. A fourth heart sound is usually present, especially if there is severe hypertrophy. In young patients, an early diastolic filling sound is frequently heard, indicating early rapid filling. In the presence of severe concomitant mitral regurgitation, the excess flow across the mitral valve may result in a diastolic flow rumble.
The classic murmur from left ventricular outflow tract obstruction is a crescendo-decrescendo murmur located primarily at the left sternal border. The murmur usually ends before the second heart sound. The murmur can radiate to the base of the heart as well as to the apex, but as opposed to valvular aortic stenosis, there is seldom radiation to the carotid arteries. Mitral regurgitation may be a separate murmur audible at the apex and is more holosystolic in nature. The presence of an aortic diastolic decrescendo murmur should suggest another disease, such as aortic valve disease or a discrete subvalvular stenosis.
Dynamic auscultation should be performed to differentiate the murmur of HCM from that of valvular aortic stenosis and mitral regurgitation. Maneuvers that decrease preload will increase the dynamic gradient and increase the intensity of the murmur (Fig. 33–7). Although the change in murmur intensity during the strain phase of the Valsalva maneuver has been proposed as a method to diagnose the dynamic murmur of HCM, this classic response may not occur in all patients. A more reliable method for diagnosing a dynamic left ventricular outflow tract obstruction is the response of the murmur to the stand-squat-stand position. From the standing position to a prompt squat, the murmur will markedly decrease in intensity, due to increases in afterload and preload. From the squatting to standing position, there will be an increase in intensity of the murmur immediately as afterload is reduced. A progressive increase in intensity of the murmur will continue for the next four to five beats as preload to the left side of the heart is reduced. Simple exercise, such as ambulation or climbing stairs, can be used to assess for augmentation of the murmur as well. Other maneuvers that are used to change the intensity of the murmur include leg-raising to increase preload (and thereby decrease the intensity of the murmur) and the inhalation of amyl nitrite to decrease afterload, increase heart rate, and increase the intensity of the murmur.
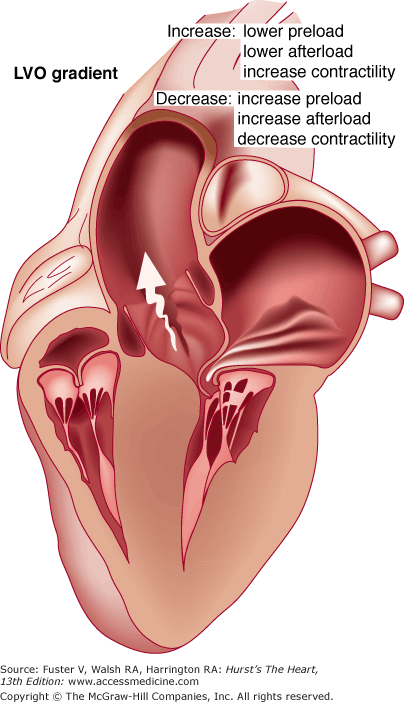
FIGURE 33–7
Schematic diagram of the left ventricle in hypertrophic cardiomyopathy during systole. There is projection of the basal septum into the outflow tract with systolic anterior motion of the mitral valve, which results in left ventricular outflow (LVO) tract obstruction. The obstruction is dynamic, dependent on the preload, afterload, and contractility of the heart.
The response of the intensity of the murmur after premature ventricular contraction or after any long pause is useful. There will be an increase in left ventricular contractility and decrease in afterload for the beat after a long pause. There will thus be an increase in intensity of the systolic murmur in obstructive HCM, whereas the murmur of organic mitral regurgitation will remain unchanged or decrease in intensity.
Diagnostic Testing
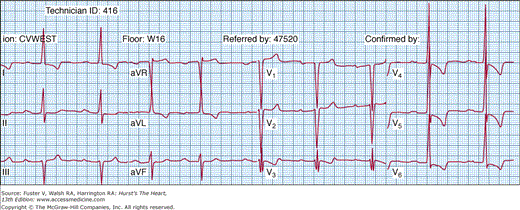
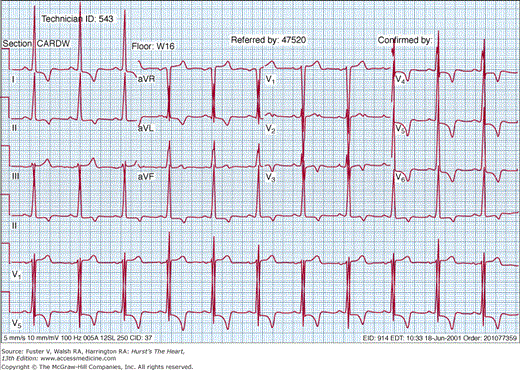
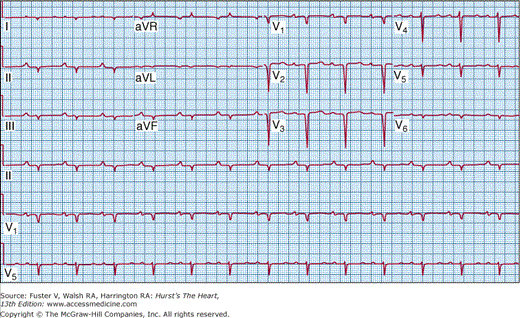
The electrocardiogram is abnormal in the majority (~95%) of HCM patients.45 A normal axis is present in 60% to 70% of patients and a left axis is present in 30%, whereas 70% to 80% of patients will demonstrate left ventricular hypertrophy on the resting 12-lead electrocardiogram (Fig. 33–8). Abnormal Q waves simulating myocardial infarction, due to a disturbance of activation of ventricular septum, are seen in 25% of patients and may appear in any lead. The electrocardiogram in patients with a variant of HCM involving primarily the apex (apical HCM) may show a distinctive pattern of diffuse symmetric T-wave inversions across the precordium (Fig. 33–9).
The basic rhythm in most patients is normal sinus rhythm, but ambulatory monitoring will demonstrate a high incidence of supraventricular tachycardia (46%), premature ventricular contractions (43%), and nonsustained ventricular tachycardia (26%).117-119 Atrial fibrillation may occur in up to 25% to 30% of the older population. Pre-excitation has also been associated with HCM.
The chest x-ray usually shows mild to moderate enlargement of the cardiac silhouette. The left ventricular contour is rounded consistent with concentric left ventricular hypertrophy. There is usually enlargement of the left atrium, and the right-sided chambers are usually normal. The presence of aortic valvular calcification or a dilated ascending aorta should raise the possibility of aortic valvular disease.
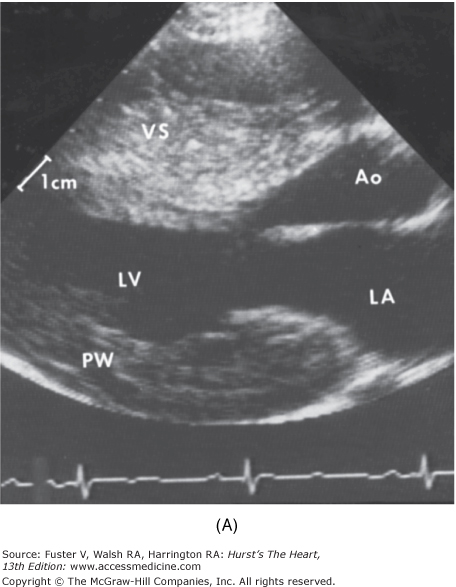
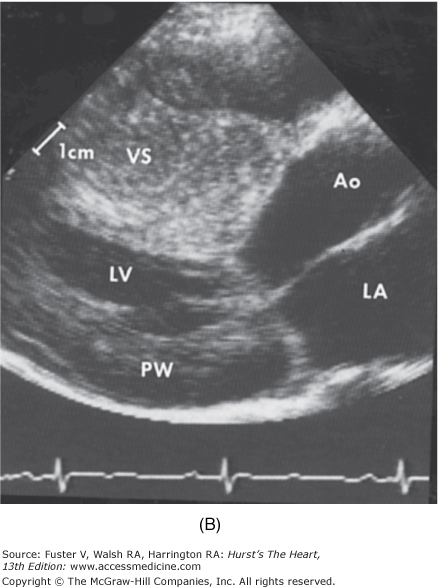
Two-dimensional and Doppler echocardiography has become the gold standard for the diagnosis of HCM.1,2 The finding of increased wall thickness in the absence of another etiology is the basis for the diagnosis of HCM (Fig. 33–10). The hypertrophy can be distributed throughout the myocardium in any pattern but commonly involves the entire ventricular septum (Fig. 33–11).52,120,121 No phenotypic expression can be considered “classic” or particularly typical of this disease. The average maximal left ventricular wall thickness in a population of HCM patients is usually 20 to 22 mm; however, 5% to 10% of patients will have maximal wall thickness in excess of 30 mm.
FIGURE 33–10
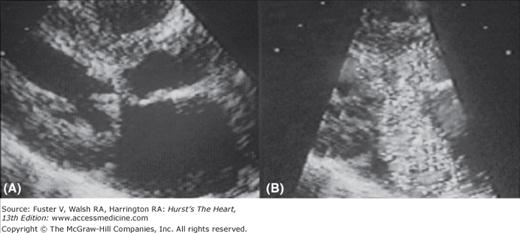
Two-dimensional echocardiogram from a patient with severe hypertrophic cardiomyopathy. There is a severe increase in left ventricular wall thickness, with a much greater increase in thickness of the ventricular septum (VS). The ratio of ventricular septal thickness to posterior wall (PW) thickness is 2.5:1. Left: Parasternal long axis view during diastole. Right: Parasternal long axis view during systole. There is systolic anterior motion of the mitral valve causing left ventricular outflow tract obstruction. Ao, aorta; LA, left atrium; LV, left ventricle.
The pattern of hypertrophy has been described as morphologically different in younger versus older patients.113 In the young population, there is classically diffuse hypertrophy of the entire septum, with a convex septal contour. In the older population, there is the appearance of a “sigmoid” septum, in which the hypertrophy is localized to the basal and midseptum. The remaining septum is concave in contour, and there is also a sharper angle of the septum with the long axis of the aorta. These differences are likely determined by the underlying genetic substrate. Patients under 50 years of age at the time of diagnosis are much more likely to have myofilament mutations than older individuals.122 Whether there are clinical, environmental, or other unique, nonmyofilament genetic abnormalities that are responsible for the sigmoid septal contour remains to be fully understood.
Genetic studies examining HCM patients with specific myofilament mutations have shown that the phenotype is not always expressed as severe thickening of the left ventricle as evidenced by those patients with identified genotype with only a mild increase of 15 to 18 mm, and still others with confirmed genotype and normal wall thickness.53,54,70,71,123 These findings may be related to the variability in the degree and age of penetrance. There are some gene mutations, such as the myosin- binding protein C mutation, in which the phenotypic expression of hypertrophy may not appear until middle age.53,54,70,71,123 Although these genotype-phenotype correlations are intriguing, it is important to acknowledge that there are numerous and important exceptions, such that the specific genotype can seldom be used to predict outcomes.
The finding of increased wall thickness on echocardiography can be seen in other abnormalities that must be considered in the differential diagnosis. Increased afterload on the left ventricle from either hypertension or valvular aortic stenosis may cause an increase in the left ventricular wall thickness. Patients with chronic renal failure, especially those on dialysis, will also present with increased wall thickness on echocardiography. Infiltrative and glycogen storage diseases such as cardiac amyloidosis, Fabry disease, and Friedreich ataxia may present with increased wall thickness and thus will mimic HCM on echocardiography.124-126 It is important to correlate the findings of increased left ventricular wall thickness on echocardiography with the electrocardiogram. If there is relatively low voltage on the 12-lead electrocardiogram in the presence of increased wall thickness, then suspicion of an infiltrative disorder (amyloidosis) should be raised (Fig. 33–12).
FIGURE 33–12
Twelve-lead electrocardiogram from a patient with amyloid heart disease. The two-dimensional echocardiogram in this patient showed a severe increase in left ventricular wall thickness, simulating hypertrophic cardiomyopathy. However, the low voltage and loss of forces on the electrocardiogram indicate that an infiltrative disease is responsible for the increased left ventricular wall thickness.
In young athletes, a physiologic form of left ventricular hypertrophy may occur that is a physiologic adaptation to intense training that may be difficult to differentiate from HCM.127,128 Elite athletes who have a dilated ventricular cavity with septal thickness <14 to 15 mm most likely have “athlete’s heart,” but the combination of these findings may not always be present. A reduction in wall thickness after cessation of training is useful to identify the athletic heart but may not be practical in all patients.127,128 Future investigation with Doppler tissue imaging, myocardial strain, and cardiac magnetic resonance imaging may provide insight into this difficult diagnostic challenge.129,130
Two-dimensional echocardiography is also the primary tool for defining the presence and severity of left ventricular outflow tract obstruction.131,132 If true dynamic obstruction is present, there will be systolic anterior motion (SAM) of the mitral valve apparatus and an observable open ventricular chamber (as opposed to complete systolic cavity obliteration). Most patients will have SAM of the anterior leaflet, but this may also occur with the posterior leaflet.133 There are frequently additional abnormalities of the mitral valve supporting structures.131,134 The exact site of the obstruction may be determined by visualizing the region of the SAM-septal contact.135 In the classic form of obstructive HCM, the obstruction will occur at the most basal portion of the septum as it projects into the left ventricular outflow tract. However, the obstruction may also extend into the left ventricle from SAM of the chordal apparatus. There may be patients with midventricular obstruction in whom a hypertrophied papillary muscle abuts against the ventricular septum.102 Two-dimensional echocardiography is useful for ruling out other causes of left ventricular outflow tract obstruction such as discrete subaortic stenosis or tunnel subaortic stenosis.136
Doppler echocardiography can be used to define the pathophysiologic processes that are present in HCM. In the presence of a dynamic left ventricular outflow tract obstruction, there will be a high-velocity “dagger-shaped” signal on continuous wave Doppler interrogation of the left ventricular outflow tract (Fig. 33–13).137 In patients with low outflow tract velocities (<3 m/s), provocation with the Valsalva maneuver, inhalation of amyl nitrite, or exercise should be performed during the Doppler study to determine whether there is a labile or latent obstruction. It is important to demonstrate the two-dimensional features of dynamic obstruction during the provocation in addition to the Doppler signal.
FIGURE 33–13
Simultaneous Doppler echocardiogram and cardiac catheterization demonstrating the presence of severe left ventricular outflow tract obstruction. The gradient between the left ventricle (LV) and aorta (Ao) at catheterization is 100 mm Hg. A continuous wave Doppler across the left ventricular outflow tract reveals a peak velocity of 5 m/s, which is consistent with a calculated left ventricular outflow tract gradient of 100 mm Hg.
Doppler color flow imaging can be used to determine the presence and severity of mitral regurgitation (Fig. 33–14).138 If the mitral regurgitation is secondary to distortion of the mitral valve apparatus from the SAM, the color jet will be directed laterally and posteriorly. In addition, the regurgitation will predominate in mid to late systole. If there is a holosystolic signal of mitral regurgitation that is directed centrally or anteriorly, then a primary abnormality of the mitral valve apparatus should be suspected. The mitral regurgitation signal by continuous wave Doppler may contaminate the outflow tract velocity signal, and care must be taken to differentiate the true outflow tract velocity from the mitral regurgitation jet (Fig. 33–15).
FIGURE 33–14
Color flow Doppler imaging of a patient with hypertrophic cardiomyopathy, severe systolic anterior motion of the mitral valve, and secondary mitral regurgitation. A. A still-frame two-dimensional echocardiogram from the parasternal view showing systolic anterior motion of the mitral valve. B. Color flow imaging demonstrating a large mosaic jet of mitral regurgitation directed posteriorly.
FIGURE 33–15
Continuous wave Doppler echocardiogram from a patient with both left ventricular outflow tract obstruction and mitral regurgitation. The continuous wave Doppler jet has a similar contour for both the left ventricular outflow (LVO) tract velocity (left) and the mitral regurgitation (MR) (right) jet. The mitral regurgitation signal is of higher velocity, and the signal is holosystolic.
Assessment of diastolic function using noninvasive methods is difficult in HCM. The transmitral flow velocity curves as determined by echocardiography cannot be used alone due to the complex interplay of relaxation and compliance abnormalities that are present in HCM.139 However, pulmonary vein flows and Doppler tissue imaging together with the transmitral flow velocity curves can improve the accuracy of estimates of left ventricular filling pressures, but the validity of these measures is less certain than in other populations.140,141
Doppler tissue imaging of the mitral annular motion is useful to evaluate longitudinal contraction of the myocardium, which is abnormal in HCM patients despite normal or supranormal ejection fraction. Abnormally low annular velocities may be useful in detecting subclinical disease (ie, those patients who carry an HCM-associated genetic abnormality but may not yet have developed the phenotypic expression of increased wall thickness).142-144 It may also be useful in distinguishing HCM from the physiologic increase in wall thickness that is observed in some athletes, who would have preserved or enhanced annular velocities.130
Transesophageal echocardiography is usually not necessary in the evaluation of the patient with HCM. In most patients, the clinically necessary anatomic and hemodynamic information can be obtained by transthoracic echocardiography. However, patients in whom discrete subvalvular stenosis or a primary abnormality of the mitral valve is suspected may benefit from transesophageal echocardiography.
Cardiac magnetic resonance (CMR) imaging provides high-resolution moving images of the myocardium and accurately determines the site and extent of hypertrophy. This can be extremely important in patients with equivocal hypertrophy and/or difficult echocardiographic images. This technique can provide better appreciation of apical hypertrophy and other less common segmental hypertrophy. SAM of the mitral valve, left ventricular outflow tract flow turbulence, mitral regurgitation, perfusion abnormalities, and intramyocardial fibrosis or scarring can also be visualized with CMR imaging.145,146 There has been increasing interest in the relationship between CMR-detected intramyocardial fibrosis and the risk for arrhythmia-related cardiac events, although the data are insufficient to conclude that this MRI finding warrants consideration as an independent risk marker.147,148
Cardiac catheterization is not required in most HCM patients because the diagnosis and determination of outflow tract obstruction can usually be made by echocardiography. In those uncommon circumstances in which there is a discrepancy between the echocardiogram and the clinical presentation, cardiac catheterization may be of benefit in demonstrating the presence and severity of a left ventricular outflow tract obstruction.
The outflow tract obstruction has been assessed by a “pull-back” pressure tracing, placing an end-hole catheter at the left ventricular apex and pulling back to the base and then into the aorta. The systolic gradient will be between the apex and base (Fig. 33–16). However, due to the small left ventricular cavity with hyperdynamic systolic function, cavity obliteration and catheter “entrapment” may occur, resulting in a falsely increased left ventricular systolic pressure. The gradient is ideally assessed by a simultaneous left ventricular inflow and left ventricular outflow (or aortic) pressure.1,2 The left ventricular inflow position avoids the problem of catheter entrapment and is best obtained by a transseptal approach.
FIGURE 33–16
Cardiac catheterization traces from a patient with hypertrophic cardiomyopathy. An end-hole catheter is placed in the left ventricular (LV) apex, pulled back to the left ventricular base, and finally into the ascending aorta. The systolic gradient of 50 mm Hg is between the left ventricular apex and left ventricular base. Note the marked decrease in pulse pressure on the first beat after the catheter has been pulled into the aorta, which is a beat following a premature contraction. There is a “spike and dome” pattern on the aortic pressure curve.
When there is little resting obstruction, provocation using the Valsalva maneuver or infusion of isoproterenol can be performed in the catheterization laboratory. The Brockenbrough phenomenon is useful for determining the presence of a latent obstruction (Fig. 33–17). After a premature contraction, there will be an increase in the contractility of the ventricle, resulting in a marked increase in the degree of dynamic obstruction. Thus there will be an increase in gradient and a decrease in the aortic pulse pressure after the pause. This is in contradistinction to a fixed obstruction in which there will be an increase in gradient from the increase in stroke volume but also an increase in aortic pulse pressure.
FIGURE 33–17
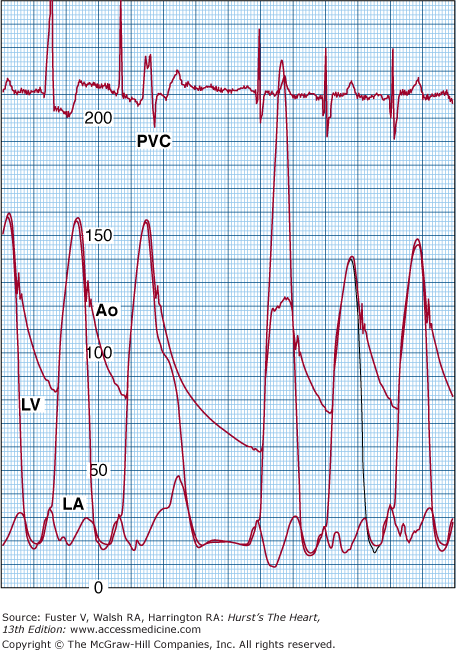
Cardiac catheterization from a patient with no resting left ventricular outflow tract obstruction. However, after a premature ventricular contraction (PVC), the left ventricular outflow tract gradient is close to 100 mm Hg. The pulse pressure of the ascending aorta (Ao) is decreased on the beat following the premature ventricular contraction. This phenomenon is termed the Brockenbrough phenomenon. LA, left atrium; LV, left ventricle.
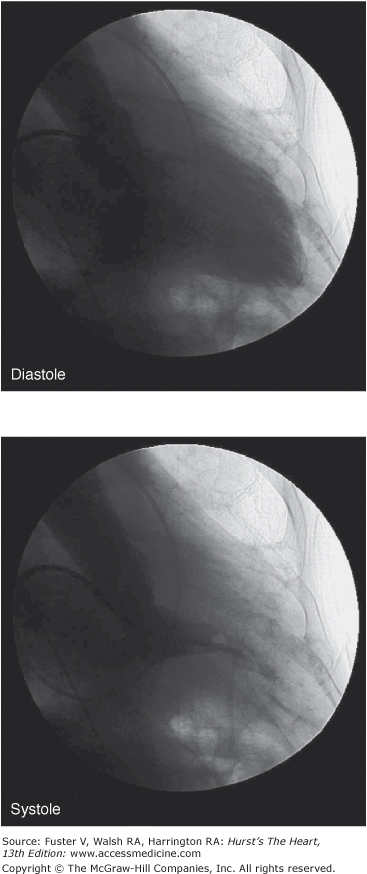
Left ventriculography will usually reveal a small left ventricular cavity size with hypertrophied papillary muscles further impinging into the cavity. Hyperdynamic systolic function will cause complete obliteration of the mid and apical cavity in systole (Fig. 33–18). In the apical variant of HCM, there will be a fixed obliteration of the apex by the hypertrophied muscle, causing a “spade-like” configuration. There may be an apical akinetic or dyskinetic pouch with “aneurysm” formation with midventricular obstruction.
Coronary angiography may be indicated if there are symptoms of angina out of proportion to the degree of obstruction or other symptoms. Epicardial coronary disease is seen in up to 25% of older patients.149 The combination of the epicardial disease and the high myocardial oxygen demand from the hypertrophied muscle may result in significant symptoms of angina and may alter outcome.150,151 Myocardial bridging is frequent, particularly in younger patients with severe hypertrophy; however, the impact of bridging on outcome is controversial.152,153
Stress testing is of limited value for the diagnosis of epicardial coronary disease in patients with HCM. The combination of the myocardial oxygen supply-demand mismatch and the small coronary arteriolar disease will result in findings of myocardial ischemia on electrocardiography and nuclear imaging, even in the absence of epicardial coronary disease. Exercise testing, however, is of value in patients with HCM. Important adverse prognostic factors include a decrease in blood pressure with exercise and/or the appearance of ventricular arrhythmias.108,154 In addition, exercise testing is useful in obtaining an objective measurement of exercise tolerance.155 Exercise is the most physiologic form of provocation to attempt to detect latent left ventricular outflow tract obstruction.156
Natural History
The natural history of HCM patients is highly variable.3,4,6,7 The early literature describing a poor prognosis with high incidence of sudden death was influenced by significant referral biases.55,56,119,157 These studies have been refuted by more recently reported mortality rates (~1% per year) from unselected regional populations.4,35-41 Indeed, HCM in the elderly has been shown to have mortality similar to control populations.115
Nonetheless, there are clearly patient subgroups at high risk for death.3-5 Death can be sudden and unexpected and may be the first manifestation of HCM, particularly in young patients.28,30,158,159 Sudden cardiac death (SCD) usually occurs in patients who have no or mild symptoms. SCD is most frequent in young adults but may also occur in the older population.40 Reports of SCD in infants and very young children are extremely rare. A substantial proportion of patients will die during or just after vigorous physical activity, and HCM has been shown to be the most common cause of sudden death among young competitive athletes.28,29,31,32,160,161 Thus, young athletes with HCM should be advised against participating in competitive sports.162-164
The mechanisms of SCD, principally ventricular tachycardia and fibrillation, have been surmised from patients experiencing implantable defibrillator discharges.165-167 However, other mechanisms including asystole, rapid atrial fibrillation, and electrical mechanical dissociation may also play a role.
There are several variables that have been associated with an increased risk of SCD.30,56,57,117,118,158,159,168 These include (1) a prior cardiac arrest or sustained ventricular tachycardia, (2) a family history of premature SCD due to HCM in a first-degree relative, (3) repetitive nonsustained ventricular tachycardia, (4) massive ventricular hypertrophy (wall thickness ≥30 mm), (5) hypotensive response to exercise, and (6) recent unexplained syncope. Electrophysiologic studies have not been shown to be of benefit for risk stratification in these patients.169,170
Other complications of HCM include infective endocarditis, systemic embolism, and atrial fibrillation. Infective endocarditis may occur in 4% to 5% of patients with HCM.45,171 The lesions are usually located at the point of opposition of the mitral valve against the septum but can involve the mitral valve and less often the aortic valve. Atrial fibrillation itself will occur in up to 30% of older patients.39,172 It usually indicates advanced disease and is associated with clinical deterioration. Acute atrial fibrillation can result in prompt hemodynamic deterioration, and immediate cardioversion to normal sinus rhythm is indicated. Systemic embolism occurs in 6% of patients and is usually associated with atrial fibrillation. Anticoagulation is recommended for patients with atrial fibrillation and HCM but will not fully eliminate the risk of stroke.