Hypersensitivity Pneumonitis
EPIDEMIOLOGY AND ETIOLOGIES
Hypersensitivity pneumonitis (HP), or extrinsic allergic alveolitis, is a spectrum of interstitial, alveolar, and bronchiolar lung diseases resulting from immunologically induced inflammation in response to inhalation of a wide variety of different materials that are usually organic or low–molecular-weight chemical antigens (or haptens), which may lead to irreversible lung damage. Despite the terms hypersensitivity and allergic, HP is not an atopic disease and is not associated with increased IgE or eosinophils. The prevalence of HP is quite variable in different populations, presumably because of differing intensity, frequency, and duration of inhalation exposure, and also probably due to host factors that have yet to be identified. Once thought to be a relatively rare disease, it is becoming more frequently recognized as awareness is of the limitations of classical diagnostic criteria has increased. Among pigeon breeders, 8% to 30% of members of pigeon-breeding clubs who participated in surveys exhibited evidence of HP, so-called pigeon breeder’s disease (Fig. 58-1). Among farmers, 0.5% to 5% have symptoms compatible with HP, so-called farmer’s lung disease. The prevalence of symptoms is lower in farms that use hay-drying methods that decrease exposure to the responsible antigens and increased after a wet summer season.
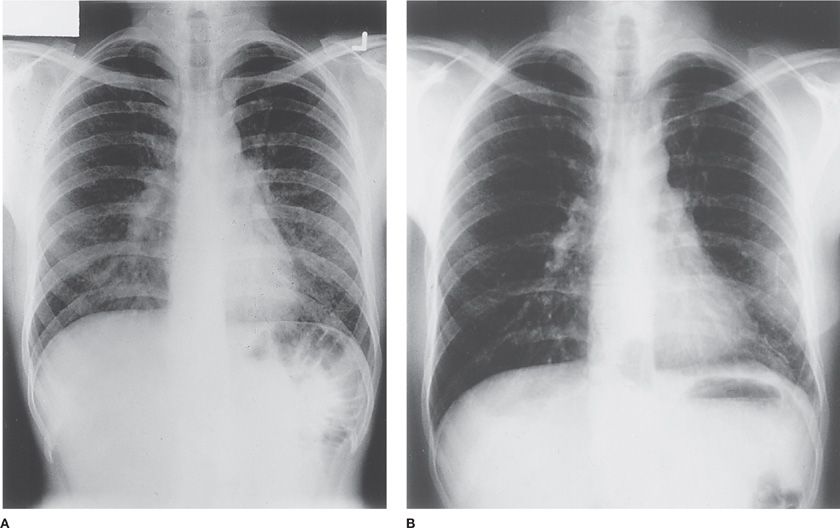
Figure 58-1 A. Chest radiograph of a patient with pigeon breeder’s disease with fever, dyspnea, and bibasilar rales. The patient had kept pigeons for 5 years and presented with fever, dyspnea, and myalgias approximately 8 hours after cleaning the pigeon coop. He had serum antibody to pigeon dropping extract. Note bilateral lower lobe 2- to 3-mm nodules. B. Chest radiograph of the same patient 2 weeks later without specific treatment. Note clearing of the lower-lobe nodules and the staples in the left chest from the open lung biopsy.
The population at risk and the season of exposure vary with the type of HP. For example, most cases of farmer’s lung disease occur in cold, damp climates in late winter and early spring, when farmers (usually male) use stored hay to feed their livestock. Pigeon breeder’s disease occurs chiefly in men in Europe and the United States but predominantly in women in Mexico, owing to differing patterns of exposure, but without a seasonal preference in either population. Bird fancier’s disease in Europe and the United States occurs in subjects who keep domestic birds and does not exhibit a predilection to either sex. Japanese summer-type HP occurs mostly in women without an occupation outside the home in June to September in warm, moist parts of the country. The disease has been reported in children as well, though rarely.
In contrast to other pulmonary diseases, there is a curious predominance (80%–95%) of nonsmokers in all examples of HP, which is substantially higher than the proportion of nonsmokers in similarly exposed individuals without HP.1 The mechanisms of this phenomenon are unknown, but could include anti-inflammatory effects of nicotine. This clinical finding suggests that the presence of active smoking may be evidence against the diagnosis of HP, although this has not been consistently observed.2
An important feature of HP is the great variability of susceptibility among exposed populations and the apparent resistance to illness of most exposed persons. Possible reasons include differences in exposure, or differences in the host response to exposure, which may be inborn and/or acquired. There are no differences in the prevalence of atopy or HLA-A, B, or C haplotypes in exposed subjects with and without HP,1 although there may be an alteration in the prevalence of several HLA-DR and -DQ alleles.3
Several case-control studies have identified single nucleotide polymorphisms (SNPs) that are disproportionately represented in patients with HP and have accordingly garnered interest as potential pathogenetic determinants. Compared with healthy controls of the same ethnic heritage, a Mexican cohort with HP was found to have SNPs in MHC class II genes encoding a family of transporters associated with antigen processing (TAP).4 Mexican HP patients also more frequently possess the KQ genotype of the PSMB8 gene, which codes for a constituent of the low–molecular-weight proteosome required for peptide processing and eventual presentation in the context of class I MHC.5 SNPs in the interleukin-6 (IL-6) gene have been correlated with higher levels of epithelial neutrophil-activating protein (CXCL-5) in the BAL fluid of HP patients,6 but the clinical implications of cytokine gene variation in this disease are undefined. An increased prevalence of a particular polymorphism in the tumor necrosis factor-α (TNF-α) promoter has been reported in patients with pigeon breeder’s disease compared with exposed subjects without pigeon breeder’s disease,7 as well as protective variants in the tissue inhibitor of metalloproteinase-3 (TIMP3) promoter in exposed subjects without pigeon breeder’s disease,8 the possible significance of which is discussed below (see Immunopathogenesis).
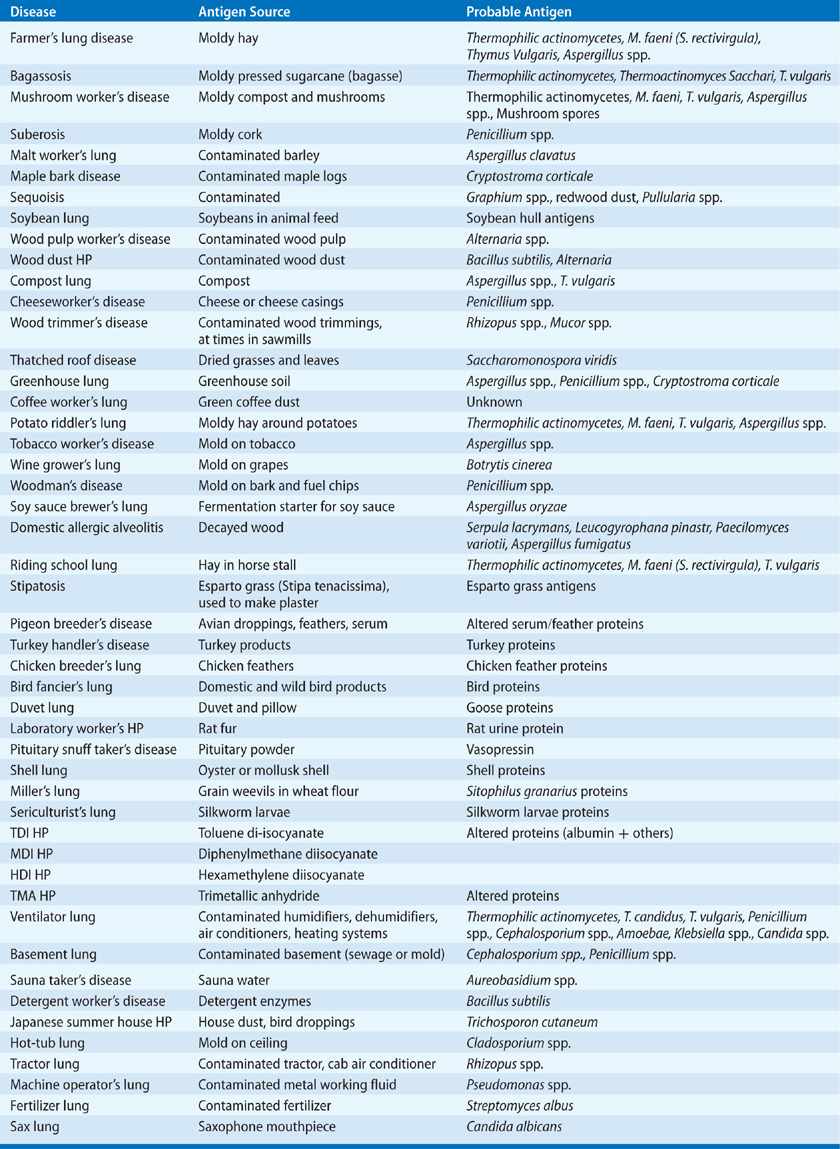
A large number of agents are associated with HP, shown in Table 58-1. Some types of HP have apparently disappeared from their originally described clinical settings (e.g., bagassosis in Louisiana) but presumably exist in areas with similar agricultural or industrial settings. In addition, other forms of HP are being newly recognized (e.g., potato riddler’s lung and machine operator’s lung). Both the disappearance of previously described examples of HP and the appearance of new examples are due to changing agricultural or industrial practices that result in changes of exposure of subjects to antigenic material that can cause HP. At the present time farmer’s lung disease, bird fancier’s disease, ventilator lung, and Japanese summer-type HP are the most commonly recognized forms of HP.
Recognition of new examples of HP usually requires a cluster of new cases with a unifying exposure history, underscoring the importance of obtaining at least a basic occupational and avocational history whenever possible. Such diligence revealed that a group of musicians developed HP from instruments contaminated with Mycobacteria in the case of trombonists9 and by molds in the case of a saxophonist.10 Use of a new metalworking fluid (MWF) led to recognition of machine operator’s lung in an auto parts–manufacturing facility due to clustering of cases and a common unusual exposure (Pseudomonas in cooling fluid). Highly sensitive genomic testing applied to MWF used in the automotive and nonautomotive industries suggests that HP risk may be associated with microbial colonization patterns,11 particularly the presence of Mycobacterium immunogenum.
CLINICAL FEATURES
The manifestations of the disease may be acute, subacute, or chronic. The stereotypical acute clinical presentation includes transient fever, hypoxemia, myalgias, arthralgias, dyspnea, and cough that occur 2 to 9 hours after exposure and resolve in 12 to 72 hours without specific treatment (sometimes longer after a particularly intense exposure). Patients exhibit tachypnea, bibasilar rales, and occasionally cyanosis. There is usually peripheral blood leukocytosis with neutrophilia and lymphopenia (without eosinophilia), and BAL neutrophilia. Subacute or intermittent disease may result from repeated exposures, and manifest as productive cough, dyspnea, fatigue, and weight loss. There may be BAL lymphocytosis, frequently (though not always) with a predominance of CD8+ T lymphocytes.
The chronic form is clinically more insidious, and patients may lack a history of acute episodes, but present with a gradual onset of cough, dyspnea, fatigue, and weight loss. Symptoms are usually present for months to years. There is typically no fever, but tachypnea and bibasilar dry rales are usually present. This form of the disease may be difficult to distinguish from idiopathic pulmonary fibrosis (IPF). Symptoms and signs of cor pulmonale are not uncommon at presentation.
The reasons for the different clinical presentations (i.e., acute, subacute, and chronic) of HP are not clear, but could include differences of intensity and duration of exposure (low-intensity long-duration exposure tending to cause chronic HP; high-intensity short-duration exposure tending to cause acute HP). This is most clearly demonstrated in HP due to bird exposure. Long-term exposure to low amounts of bird antigens is associated with chronic HP. Pigeon breeder’s disease has different presentations in different geographic areas, manifesting as an acute HP in some and chronic HP in others. Intermittent exposure of pigeon breeders to large amounts of pigeon antigens in the United States and Europe is associated with acute disease and a good prognosis, whereas chronic exposure to a few household pigeons in Mexico is associated with chronic disease and a much poorer prognosis. In the United States and Europe, pigeon breeders keep their animals in an enclosure separate from their living areas, which they visit periodically so that exposure is intermittent. In Mexico, birds are often kept in living quarters so that exposure is constant. It is of interest that bird antigens can persist in a room for substantial lengths of time (>18 months) after removal of the birds,12 so that Mexicans with pigeon breeder’s disease might be exposed to pigeon antigens for prolonged periods even after removal of the pigeons. Therefore, pigeon breeder’s disease in Mexico resembles bird fancier’s disease in the United States and Europe in type of exposure, clinical presentation, and prognosis. It differs greatly from the acute HP that characterizes the pigeon breeder’s disease in the United States and Europe. Since the relevant antigens are similar in these two examples of bird-associated HP, it is likely that the type of exposure, and not the antigen characteristics, determines clinical presentation and prognosis. The recognition of a new example of HP is usually associated with the acute form, which is likely related to the relative ease in making the association of acute disease and an acute exposure.
The previous discussion indicates that HP, and particularly chronic HP, may be more prevalent than is readily apparent and may often be confused with other diseases, such as chronic bronchitis or IPF. The latter may be particularly important because detailed histories are not always obtained from patients with IPF, the serum antibody levels to the agents responsible for HP tend to wane after cessation of exposure, and chest high-resolution computed tomography (CT) scans of chronic HP can resemble those of IPF.
RADIOGRAPHIC FEATURES
The chest radiographs of patients with acute and chronic HP differ significantly. In acute HP, chest radiographs demonstrate diffuse poorly defined nodular radiodensities, often with areas of ground-glass radiodensities or occasionally even consolidation. These radiodensities tend to occur in the lower lobes and spare the apices. Linear radiodensities (presumably representing areas of fibrosis from previous episodes of acute HP) may also be present. The nodular and ground-glass densities tend to disappear after cessation of exposure, so the chest radiograph may be normal after resolution of an acute episode of HP (Fig. 58-2). High-resolution CT scans often demonstrate ground-glass densities better than chest radiographs and at times reveal diffusely increased pulmonary radiodensities. They may also become normal after resolution of an acute episode. Pleural effusions or thickening, calcification, cavitation, atelectasis, localized radiodensities (coin lesions or masses), and intrathoracic lymphadenopathy are rare.
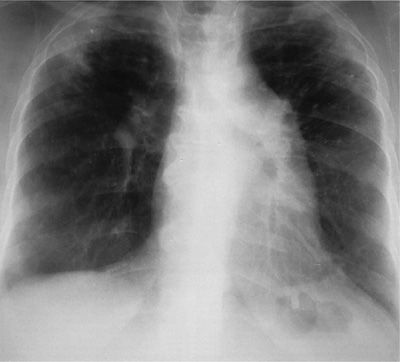
Figure 58-2 Chest radiograph of a patient with bird fancier’s disease who presented with progressive dyspnea and weight loss. She had kept two to three parakeets in her home for 15 years and did not notice episodic fever or acute dyspnea. She had positive serum precipitins to parakeet serum, severe restrictive disease, and resting hypoxemia. Note the diffuse radiodensities, loss of volume of the upper lobes, and pulmonary hypertension.
In chronic HP, chest radiographs are notable for diffuse linear and nodular radiodensities, with sparing of the bases and upper-lobe predominance, and volume loss (Fig. 58-3). Pleural effusions and thickening are very unusual, although subcutaneous emphysema (presumably as a consequence of pleural rupture due to bronchiolitis and lobular overinflation) has been reported.
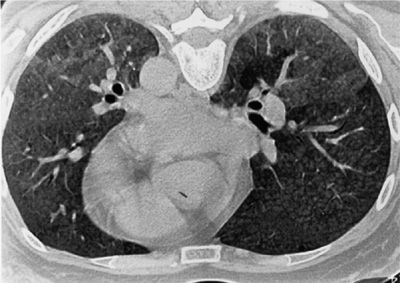
Figure 58-3 High-resolution CT scan of a nonsmoking patient with exposure to both birds and shells who presented with progressive dyspnea and weight loss and had hypoxemia and a restrictive ventilatory defect. Note the diffuse nodular radiodensities in the lower lobes, with areas of ground-glass densities posteriorly.
High-resolution CT scans of patients with chronic HP demonstrate several patterns. Most commonly there are multiple centrilobular nodules 2 to 4 mm in diameter throughout the lung fields, with some areas of ground-glass radiodensities, especially in the lower lobes (Fig. 58-4). Unlike sarcoidosis, the nodules are seldom attached to the pleura or bronchovascular bundles, and the border between the nodules and the surrounding lung is well demarcated. There are also well-delineated areas of increased radiolucency, which are presumably overinflated pulmonary lobules subserved by partly occluded bronchioles. The ground-glass densities and micronodules tend to resolve after cessation of exposure. Although these findings are suggestive of HP, they are found in only a subset (50%–75%) of patients with HP, and high-resolution CT scans of the lungs of patients with HP can resemble those of patients with IPF. In cases of chronic avian-related HP, honeycombing and airspace consolidation on high-resolution CT scans are independently associated with increased mortality risk, even after controlling for spirometric and demographic variables.13 Emphysematous abnormalities are also commonly detected by high-resolution CT scans in nonsmoking patients with farmer’s lung disease.
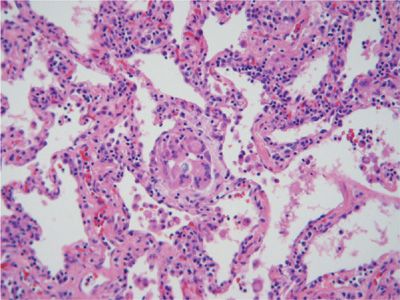
Figure 58-4 Low power view (20×) of H&E-stained section of surgical lung biopsy from a patient with bird fancier’s disease. There are nonspecific interstitial mononuclear inflammation and loosely formed granulomatous lesions.
 LABORATORY FINDINGS
LABORATORY FINDINGS
Patients with acute HP often have a peripheral blood leukocytosis with neutrophilia and without eosinophilia. Prominent cellular abnormalities may also be seen in their BAL fluid, which may be useful in supporting the diagnosis of HP. At time points greater than 5 days after the last exposure, a two- to fourfold increase in BAL fluid leukocytes and lymphocytosis (typically 30%–70% of total cells) are frequently noted. In most instances of HP, the BAL fluid lymphocytes are virtually all CD3+ (T lymphocytes), with a relative increase of CD8+ cells, so that the CD4:CD8 ratio is usually less than 1 (normally 2–2.5, as in peripheral blood).14 This profile varies considerably with the stage of disease. Indeed, only 33 of 98 HP patients (34%) in one case series displayed a lymphocytic CD8+ alveolitis.15 Findings of a higher CD4+:CD8+ ratio, fewer γδ T cells, and more terminally differentiated memory CD4+ and CD8+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree