Epidemiology
Acquired immunodeficiency syndrome (AIDS) was first recognized in 1981 and is caused by human immunodeficiency virus (HIV-1). HIV-2 causes a similar illness to HIV-1 but is less aggressive and has so far been restricted mainly to western Africa. HIV/AIDS is acquired through exposure to infected body fluid, particularly blood and semen; the most common modes of spread are sexual, parenteral (blood or blood product recipients, injection drug users, and occupational injury) and vertical (mother to fetus).
HIV/AIDS is now the second leading cause of death in the world, with a global prevalence of 0.8%. More than 33 million people are living with HIV/AIDS, with 2.7 million new infections and 2 million AIDS-related deaths reported in 2007.1 The vast majority of deaths have occurred in sub-Saharan Africa, where more than 13 million children have been orphaned and approximately two-thirds of the global HIV/AIDS burden exists.1 In the United States, more than 1 million people are HIV infected, and infection rates are increasing rapidly in many parts of the world, notably south Asia and eastern Europe.2
Many cultural and social factors determine regional patterns of HIV/AIDS disease and associated infections.3 In the United States and northern Europe, the epidemic has predominantly been in men who have sex with men. In southern and eastern Europe, Vietnam, Malaysia, northeast India, and China, the incidence has been greatest in injection drug users, but in Africa, the Caribbean, and much of southeast Asia, the dominant routes of transmission have been heterosexual and from mother to child (vertical).
The epidemic in industrialized nations is also changing. In these countries, heterosexual transmission is now the dominant route of infection. For example, in the United Kingdom, the number of new HIV/AIDS diagnoses among heterosexuals has outnumbered those among homosexual and bisexual men since 1999; 54% of new infections in 2005 were acquired heterosexually. The disease is increasingly seen in women, and in the United States, the proportion of female HIV/AIDS patients rose from 7% in 1985 to 23% in 1998.4
HIV-Related Cardiovascular Disease
Various heart diseases have been documented in up to 40% of autopsy cases and during life by echocardiography in approximately 25% of patients with AIDS (category C disease; see below). However, many of these pathologic lesions are mild, and HIV-related heart disease probably causes symptoms in fewer than 10% and death in fewer than 2% of all patients with HIV infection. Common cardiovascular manifestations of HIV infection are listed in Table 93–1.
| Manifestation | Description |
|---|---|
| Pericardial effusion | Idiopathic Infectious (viral, bacterial [especially tuberculous], and fungal) Neoplastic (Kaposi sarcoma, and NHL) |
| Heart muscle disease | Myocarditis (idiopathic or lymphocytic, specific infections, toxins) Dilated cardiomyopathy and LV dysfunction |
| Endocarditis | Marantic (nonbacterial thrombotic endocarditis) Infective |
| Tumors | Kaposi sarcoma Lymphoma |
| RV dysfunction or pulmonary hypertension | Primary Secondary (recurrent chest infections, thromboembolism) |
| Premature atherosclerosis and coronary artery disease | |
| Adverse drug effects | Hyperlipidemia Proarrhythmia |
| Vascular disease | |
| Autonomic dysfunction |
At the beginning of the epidemic, heart muscle disease (cardiomyopathy) was the dominant cardiac complication of HIV infection in the developed world. In contrast, tuberculous pericarditis and cardiomyopathy were and still remain important cardiac manifestations of the disease in Africa.5,6 Combined highly active antiretroviral therapy (HAART) (usually two nucleoside reverse transcriptase inhibitors [NRTIs] in combination with one or two protease inhibitors)7 has changed the pattern of disease in developed countries, where premature coronary artery disease (CAD) and other manifestations of atherosclerosis are emerging as the most common cardiovascular disorder. This is partly attributable to HAART-induced metabolic problems, particularly insulin resistance and hyperlipidemia, but also reflects a high prevalence of conventional cardiovascular risk factors such as smoking. Cardiovascular problems associated with advanced immunodeficiency, such as heart muscle disease, pericardial effusion, and pulmonary hypertension, continue to predominate in resource-poor countries, where fewer than 5% of patients have access to antiretroviral drugs.5,6
Natural History and Biology of HIV Infection
HIV is a single-stranded RNA retrovirus from the Lentivirus family that invades cells containing specific membrane receptors and incorporates a DNA copy of itself into the host’s genome. Immune deficiency is the result of virus and immune-mediated destruction of CD4 lymphocytes caused by continuous high-level HIV replication. The reduction in the number of CD4 cells circulating in peripheral blood is tightly correlated with the plasma viral load. Both can be monitored and are used as measures of disease progression. Virus-specific CD8 cytotoxic T-cell lymphocytes develop rapidly after infection and can lyse infected CD4 cells. They play a crucial role in controlling HIV replication after infection and may therefore determine the rate of disease progression.
Any depletion in CD4 cells renders the body susceptible to opportunistic infections and oncogenic virus-related tumors. The predominant opportunist infections seen in HIV/AIDS disease are intracellular parasites (eg, Mycobacterium tuberculosis) and pathogens susceptible to cell-mediated rather than antibody-mediated immune responses.
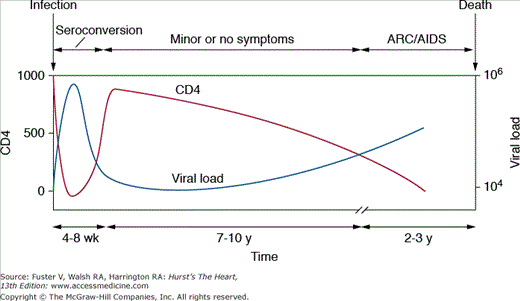
After the initial infection, there is a dormant period before symptoms and disease supervenes (Fig 93–1). In the absence of antiretroviral therapy (HAART), the average length of life after infection is approximately 10 years.8 In contrast, HAART can essentially eliminate HIV viremia and transform the patient’s prognosis to one of a chronic disease with associated organ-specific illnesses that require specialized treatment.
Primary infection is usually established 2 to 6 weeks after exposure.9 Most (70%-80%) patients experience a self-limiting illness, similar to infectious mononucleosis, characterized by fever, fatigue, pharyngitis, lymphadenopathy, and maculopapular rash. In many patients, the illness is mild and only identified by retrospective enquiry at later presentation. More than 95% of patients seroconvert (ie, become HIV positive) within 6 months. This usually coincides with a surge in plasma HIV RNA levels to more than 1 million copies/mL (peak between 4 and 8 weeks) and a decrease in the CD4 count to 300 to 400 cells/mm3. Symptomatic recovery is accompanied by an increase in the CD4 count and a decrease in viral load; nevertheless, the CD4 count rarely recovers to its original value (see Fig 93–1).
A simple Centers for Disease Control and Prevention (CDC) classification has been used to describe the subsequent phases of disease (Table 93–2). Whereas premature coronary disease, infective endocarditis, and drug-related problems may supervene at any time, pericardial effusion and heart muscle disease are usually only seen in late stage (CDC Category C) disease.
| Category | Description |
|---|---|
| Category A | Asymptomatic patients; progressive lymphadenopathy |
| Category B | Symptomatic patients without AIDS-defining illness |
| Category C | Symptomatic patients with AIDS-defining illness |
Viral replication takes place in the lymphoid tissue throughout this stage; there is sustained viremia, and the CD4 count decreases steadily (typically by between 50 and 150 cells/y). Although there may be persistent generalized lymphadenopathy, the patient remains well.
The median interval from infection to the development of symptoms is around 7 to 10 years. A variety of diseases known as AIDS-related complex conditions (eg, oral hairy leukoplakia, weight loss, night sweats, and chronic diarrhea) may supervene but are not AIDS defining.
AIDS is defined by the development of one or more specified opportunistic infections, tumors, and other conditions. These include esophageal candidiasis, cytomegalovirus CMV retinitis, pulmonary or extrapulmonary tuberculosis, Kaposi sarcoma, and HIV/AIDS-associated dementia. Most forms of HIV-related heart muscle disease and pericardial effusion occur at this stage.
Cardiovascular Assessment of HIV/AIDS Patients
It seems likely that as survival increases with improved therapy,7 more HIV/AIDS patients will be seen by cardiologists, and indeed the number of reports of cardiovascular problems in HIV/AIDS is increasing.10-12 Nonetheless, cardiovascular disease in individuals with HIV/AIDS is also becoming increasingly recognized in the developing world perhaps as antiretroviral therapy becomes more readily available there.5
Heart disease may be overlooked in HIV-positive patients because symptoms of breathlessness, fatigue, and poor exercise tolerance are frequently ascribed to other conditions associated with HIV infection.10 Echocardiographic assessment of HIV/AIDS patients is extremely useful13 and can be used easily to identify those cardiac conditions common in HIV-positive patients that may be associated with a poor outcome, including pericardial effusion,5 left ventricular (LV) systolic dysfunction or heart muscle disease,10 and intracardiac masses. Echocardiography may also provide useful information on the appearance of the right ventricle (RV), provide an indirect assessment of pulmonary pressures, and reveal regional wall motion abnormalities suggestive of CAD.
It has been suggested that any HIV-positive patient who is at high risk of developing or who demonstrates any potential clinical manifestation of cardiovascular disease should have a baseline echocardiogram performed. Thereafter, serial echocardiography should be performed every 1 to 2 years.14 Unfortunately, logistical limitations make it unlikely that these goals can be achieved in the developing world. Similarly, it may be justifiable to perform a baseline echocardiographic study at the time of diagnosis of HIV/AIDS with annual to biannual examination of asymptomatic patients (Table 93–3).15 Clearly, more aggressive monitoring may be guided by the cardiologist on discovery of abnormalities or in those patients with significant, potentially cardiotropic viral infections or unexplained pulmonary symptoms.
| Possible baseline assessment at time of diagnosis of HIV infection |
| Baseline assessment and monitoring every 1 to 2 years of patient with: |
| Clinical manifestation of possible cardiac involvement |
| Unexplained dyspnea or hypoxia |
| Third heart sound or inappropriate tachycardia |
| Raised JVP |
| Peripheral edema or right heart failure |
| Radiographic evidence of cardiomegaly |
| Viral coinfection |
| Cytomegalovirus |
| Epstein-Barr virus |
| Coxsackie virus |
| Adenovirus |
| History of preexisting cardiac disease |
| LV systolic dysfunction (all cause) |
| Valvular heart disease |
| Suspicion of infective endocarditis in injection drug abuse |
| High-risk HIV patients with: |
| Wasting |
| Encephalopathy |
| CD count <100/AIDS |
| Potentially cardiotoxic medication (see later in chapter) |
| Multiple hospitalizations |
| Possible monitoring every 1-2 y of asymptomatic HIV-positive patients |
| Frequent assessment of HIV-positive patients with cardiovascular involvement (as guided by cardiologist) |
HIV/AIDS and the Pericardium
Pericardial effusion and pericarditis were the commonest cardiac abnormalities found in early HIV/AIDS autopsy studies and remain a significant problem in Africa, where the largest number of HIV/AIDS patients are found.5,6 Pericardial effusion was found in up to 38% of patients, particularly in association with generalized fluid retention and advanced disease.16 Small effusions are still found frequently in patients with heart failure or malignant infiltration, but cardiac tamponade may occur rarely. The finding of cardiomegaly on chest radiography should prompt early echocardiographic assessment.17
Clinically significant pericardial effusions are usually caused by viral or bacterial infection or malignant infiltration, particularly with Kaposi sarcoma or non-Hodgkin lymphoma (NHL). In Africa, pericardial effusion itself is suggestive of HIV infection, and up to 72% of African patients with serosanguineous effusions have been found to be HIV positive.5,6 Pericarditis caused by M. tuberculosis or Mycobacterium avium–intracellulare infection is a pressing problem in Africa but has also been reported as the first manifestation of AIDS in Europe.
Other unusual pathogens, including Nocardia asteroides and herpes simplex virus, should be considered along with CMV, which remains prevalent in the HIV/AIDS population often without a definite anatomical site of infection. Appropriate antituberculous and antiviral therapies may therefore be helpful in this situation.
Surgical intervention is not always beneficial in AIDS patients with large pericardial effusions.18 However, no data are available on the long-term outcome of such measures in patients at an earlier stage of HIV/AIDS. Pericardiocentesis and pericardiectomy was used to treat a Staphylococcus aureus pericardial tamponade in an HIV-positive drug user, who remained well for more than 5 years.19 Culture of pericardial biopsy or fluid from symptomatic effusions may also be also useful in identifying treatable opportunistic infections or malignancy.
HIV/AIDS and the Myocardium
Numerous pathologic studies confirmed the presence of varying histologic patterns of lymphocytic myocarditis in HIV/AIDS patients,16 although many do not fulfill the Dallas criteria20 formulated to secure the histopathologic diagnosis (see Chap. 35). Clinical correlation with myocarditis has been described in AIDS series,21 and opportunistic infections were prominent comorbid conditions. However, interstitial mononuclear infiltrates have also been reported in other forms of cardiomyopathy and noncardiomyopathic conditions.22 The apparent difference in the prevalence of myocarditis in different studies may relate to clinical factors, sampling errors, and possibly even the later effects of HAART. As such, estimates of the prevalence of myocarditis in HIV/AIDS varies from 53%23 in the pre-HAART era to much lower levels today in the developed world, with the conditions remaining prevalent and problematic in the developing world.5
Myocarditis can be precipitated by a variety of viral infections, and the inflammatory reaction can progress even after virus is no longer evident in the heart. An immune reaction, either to viral antigen or to altered myocardial protein, may precipitate myocardial necrosis and inflammatory cell infiltration.24 However, simple histopathologic methods alone may be insufficient to exclude the diagnosis of myocarditis in AIDS patients. Infiltrating CD8 and CD45 lymphocytes have been found in association with increased MHC class I antigen expression in histologically normal endomyocardial biopsies from HIV-positive patients with cardiac failure.25
It remains possible that a subgroup of AIDS patients may have immune-mediated heart disease despite normal biopsies, and an inflammatory process remains the likeliest substrate for the development of cardiac dysfunction in HIV-positive patients. There are several hypotheses regarding the cause of myocarditis in AIDS, including primary HIV myocarditis, secondary HIV myocarditis, opportunistic infection, and autoimmunity.
The virus HIV per se has neither been universally accepted nor unambiguously proven a causative agent of myocarditis in AIDS. HIV-1 gains entry into cells through binding between its envelope glycoprotein group 120 and CD4 receptors, which are found on T4 (helper) lymphocytes and some other cell types. Although HIV can infect monocytes or macrophages and myocardial interstitial cells, evidence proving that HIV can infect human cardiac myocytes, which do not possess CD4 receptors, is less clear.
Some reports of cardiac infection by HIV26 used in situ hybridization, which did not require extraction of target sequences and thus preserved the histologic architecture. However, the cell responsible for hybridization with HIV probes would be masked by the dense silver reaction that occurs as part of that technique. Accordingly, precise localization and definite assignment of the hybridization signal to the cardiac myocyte was not possible. Cell culture and polymerase chain reaction (PCR) were equally limited in that such tissue samples could easily be contaminated by the patient’s own infected blood cells. A single HIV contaminant virion for example from a nearby infected interstitial cell would confuse interpretation. Similarly, examination of hearts from nonhuman primates (macaques) infected with simian immunodeficiency virus (SIV) has been accomplished using similar methods. These studies confirmed that SIV infected the cardiac monocytes rather than actual myocytes.27
Despite this, HIV gene sequences have been detected by PCR in microdissected endomyocardial biopsies from HIV-positive patients, some of whom had cardiac symptoms.28 HIV has also been shown to gain entry into the human fetal cardiac myocyte by ingestion through a specific Fc receptor, and it remains possible that this or other unidentified mechanisms may promote HIV entry into the myocyte and facilitate a primary HIV myocarditis.29
Immune responses are implicated in Chagas’ cardiomyopathy in which noninfected myocytes are damaged by the host response to Trypanosoma cruzi (see Chap 35). Interstitial lymphocytes and macrophages may form contact with myocytes, causing a focal loss of basement membrane through a local reaction.30 A similar process may be involved in the pathogenesis of HIV myocarditis. Proteolytic enzymes released through HIV replication in the interstitium could also damage myocytes. Such “innocent bystander destruction”31 may be particularly relevant to the myocardium because increased numbers of infected interstitial cells have been found in HIV-positive subjects with active myocarditis.
The HIV envelope glycoprotein group 120 can induce tumor necrosis factor (TNF)-α expression from macrophages and has been shown to enhance interleukin (IL)-1β–induced nitric oxide production in neonatal rat cardiac myocytes.32 Cytokine IL-6, which has some effect on immune response and viral replication in murine myocarditis models, has been found in excess in a small number of HIV-positive patients with biopsy-proven myocarditis.33 Therefore, just as cytokines may have a role in the development of congestive heart failure in absence of HIV/AIDS, they may also be important in the course of HIV/AIDS myocarditis and cardiomyopathy.
Autopsy has confirmed a variety of opportunistic infections of the myocardium in patients with AIDS. Infectious agents have included Toxoplasma gondii in the hearts of adults and children, Cryptococcus spp., CMV, Candida spp., Pneumocystis carinii, Microsporidium spp., Histoplasma capsulatum, atypical mycobacteria, and Aspergillus organisms involving the myocardium. Most of these have been part of a disseminated infection and are infrequently associated with localized myocarditis.
Acute Chagas’ myocarditis in patients with AIDS also has been reported and may be associated with a more frequent rate of myocarditis in up to 30% of cases in AIDS patients.34 However, a clear causative link between opportunistic infection and myocarditis in AIDS has yet to be established.
Myocarditis can be diagnosed clinically based on symptoms and physical findings, although this is often difficult in HIV/AIDS patients. The symptoms are protean and include fatigue, dyspnea, and pleuritic chest pain, which may wrongly be ascribed to other conditions. The finding of an unexplained tachycardia, a third heart sound, or a friction rub should alert the physician to the possibility of the myocarditis and guide investigation.
The electrocardiogram (ECG) may be helpful, possibly demonstrating nonspecific conduction defects, repolarization abnormalities, and ST–T-wave changes. The chest radiograph may be normal or suggest cardiac enlargement with pulmonary congestion. Echocardiography is usually nondiagnostic but may show hyperdynamic LV function in HIV-positive children with myocarditis35 or occasionally LV dyskinesia in adult HIV/AIDS patients. The utility of a myocardial biopsy remains unclear in this setting. Sampling errors may reduce the diagnostic yield from this invasive procedure, and finding a treatable cause of biopsy-proven myocarditis is rare.
Dilated cardiomyopathy as a complication of HIV infection was first described in 198636 and was identified frequently thereafter. The pathogenesis remains obscure, but features of HIV/AIDS-related heart muscle disease are similar to idiopathic dilated cardiomyopathy in HIV-negative individuals, and an association between cardiac dysfunction and the lymphocytic myocarditis reported in HIV/AIDS postmortem series seems plausible. Isolated LV dysfunction in HIV/AIDS patients may resolve spontaneously, suggesting a self-limiting myocarditis. This reflects current thinking on the pathogenesis of cardiomyopathy in general. However, there is only a loose correlation between the histologic abnormalities found in HIV/AIDS studies and clinical evidence of LV dysfunction.
Studies involving HIV-negative control groups made up of patients with hematologic malignancy, high-risk lifestyles, or matched HIV-positive patients suggest that cardiomyopathy in HIV/AIDS is not the result of high-risk activities or a nonspecific manifestation of a chronic illness.37,38 However, it is reasonable to assess the impact of and role of any comorbid conditions or risk behavior before associating HIV/AIDS as the solitary causative factor for cardiomyopathy in AIDS. The differential diagnosis of cardiomyopathy in HIV/AIDS includes LV dysfunction secondary to ischemic heart disease, diabetes or hypertension, hypersensitivity reactions to drugs or foreign injected material, and coronary spasm secondary to cocaine use.39
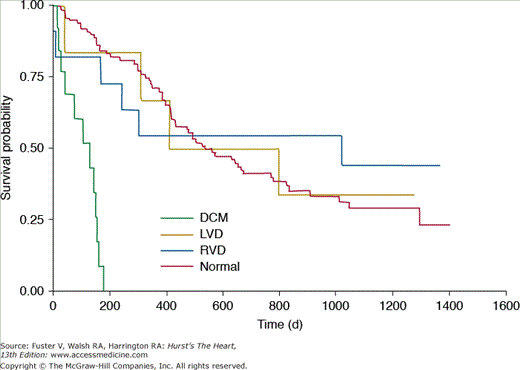
The prevalence of heart muscle disease appears to be around 4.4% for cardiomyopathy and 6.4% for isolated LV dysfunction, and these conditions may cause symptoms in up to 5.5% of HIV/AIDS patients.38 Cardiomyopathy is an ominous harbinger that is associated with poor survival in HIV/AIDS patients compared to similar patients with structurally normal hearts. This poor outlook remained true even after correcting for CD4 counts (Fig. 93-2). A 1-year consecutive enrollment study of patients admitted to the intensive care unit in an urban center revealed 6% of admissions with HIV/AIDS had echocardiographically documented cardiomyopathy with a short-term mortality rate of 25%.40
The mechanisms for the development of LV dysfunction or cardiomyopathy and myocarditis in AIDS remain unclear. In addition to the role of HIV and its proteins, lymphocytic myocarditis and cytokines, the contributions of autoimmune responses, illicit and prescribed medications, nutritional deficiencies, and other factors also appear to be pathogenetically or pathophysiologically important.41
Many autoimmune processes have been described in association with HIV/AIDS. Although some of these may be the result of opportunistic infection, HIV infection may trigger autoimmune phenomena in susceptible patients.42
The significance of some autoantibodies such as antineutrophil cytoplasmic autoantibody or antiphospholipid antibodies in HIV/AIDS remains unclear. However, the presence of such antibodies with hypergammaglobulinemia and elevated circulating immune complexes suggests that an as yet undefined autoimmune process may take place in HIV-positive patients.43
Autoantibodies against β-myosin have been identified in HIV-positive patients with cardiomyopathy and histologically proven active myocarditis. Antibodies to α-myosin, which are more highly cardiac specific, have also been found more frequently and in higher levels in HIV/AIDS patients with heart muscle disease than in those with normal hearts or hearts of HIV-negative control subjects.37
These findings support a possible autoimmune process in the pathogenesis of HIV/AIDS heart muscle disease. In experimental models, susceptible mice developed cardiomyopathy with antimyosin antibodies after exposure to Coxsackie B3 infection or immunization with α-myosin. Common, cardiotropic viruses could facilitate development of cardiac autoimmunity in HIV-positive patients by modifying myocyte surface antigens. CMV infection is a common opportunistic infection in AIDS patients, but it has been described only infrequently as a cause of myocarditis in HIV/AIDS.16 Although this might suggest that CMV is not strongly implicated, in situ hybridization studies have identified transcripts of CMV-specific DNA within the myocytes of HIV-positive patients with myocarditis and cardiomyopathy in the absence of typical histologic features such as inclusion bodies.25
In this way, CMV or some other factors such as circulating HIV viral proteins could be responsible for the ongoing inflammation and cardiac injury seen in many cases of HIV/AIDS myocarditis. Cardiac dysfunction and heart muscle disease have been associated with the expression of HIV proteins Tat and Vpr in transgenic mice.44-46 These proteins may in turn be implicated in the oxidative imbalance found in HIV/AIDS.47
Both HIV-1 infection per se and antiretroviral therapy, particularly NRTIs, may have a negative impact on myocardial function.48,49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree