Helminthic Diseases of the Lungs
Parasitic helminths are a distinct group of infectious agents that are among the most prevalent causes of morbidity and mortality in humans worldwide. Billions of people harbor parasitic worms. People with helminthic infections of the lungs often seek medical advice because of one or more common chest complaints—cough, pain, or breathlessness. They may also have unexplained laboratory abnormalities, including eosinophilia or pulmonary nodules. They can pose a diagnostic challenge, particularly in areas where helminthic infections are not endemic. More common causes of chest complaints have to be excluded, a history of residence in certain geographic locations or of dietary or other exposures has to be elicited, and the proper procedures for making the diagnosis of helminthiasis selected.
The helminths that parasitize humans include the nematodes (roundworms) and the platyhelminthes (flatworms), with the flatworms divided into the cestodes (tapeworms) and the trematodes (schistosomes and other flukes). The biology of each of these groups is distinct. Ectoparasites (e.g., the Annelida, such as leeches, ragworms, or earthworms) are uncommonly associated with lung disease and are not discussed here.
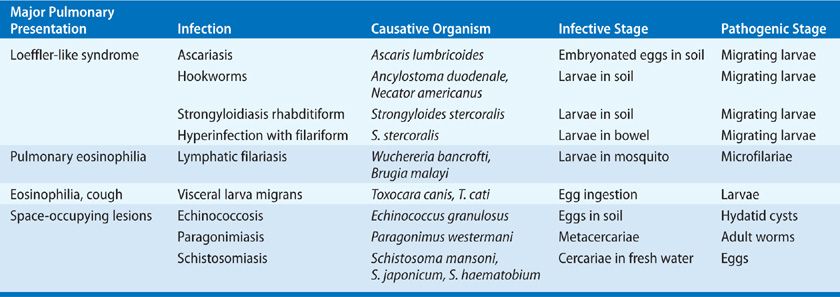
In humans, worms produce a variety of pulmonary parenchymal and vascular diseases (Table 137-1). Familiarity with the biologic behavior of each organism is essential for proper diagnosis and treatment because several stages in the life cycle of the parasite are typically found in humans, and pulmonary lesions can occur at different stages, depending on the infecting parasite.
TABLE 137-1 Pulmonary Parenchymal and Vascular Diseases Produced by Worms
BIOLOGY AND IMMUNOLOGY
Worms are multicellular organisms that vary substantially in size—from a few millimeters to several meters. They are covered by a tegument cuticle that protects them from the environment. Reproductive organs – both sexual and hermaphroditic – take up a large portion of the body. They are among the most developed and elaborate of human parasites, and their parasitic capabilities are such that they often inhabit more than one host and survive different hostile environments. Despite their relatively large adult size, the infective stages of the worms invade human tissues by ingestion, penetration of skin, or the bite of insect vectors. Furthermore, parasitic helminths have developed a myriad of mechanisms by which they may evade the defense system of the host.
The life cycle of the helminths includes an egg form, several larval stages, and an adult form. Human infection is by ingestion of the eggs or larvae, penetration of the skin by larvae, or insect transmission of larvae. Humans may be the only host (in whom both development and reproduction of the parasite occur), an accidental host (in whom neither development nor reproduction occur), an intermediate host (in whom only asexual development occurs), or a definitive host (in whom sexual reproduction occurs). A basic biologic generalization about helminthic infections is that the worms, as a rule, cannot multiply within the mammalian host. This phenomenon is important for understanding the dynamics of helminthic infection and the relationship between the intensity of a particular worm load and its pathologic consequences to the host. However, there are exceptions to this rule. For example, Strongyloides stercoralis and Echinococcus granulosus can increase their numbers within a host, even though the host is not exposed to additional infective forms. This ability of S. stercoralis to autoinfect the same subject is of considerable clinical significance, especially in immunosuppressed patients, in whom it can lead to a fatal hyperinfection. A different example is that of echinococcosis, where dissemination is usually a consequence of leakage or rupture of a hydatid cyst, which allows its contents to initiate similar lesions elsewhere.
Another biologic characteristic of worm infections is the association with tissue and peripheral blood eosinophilia and increased levels of immunoglobulin E, similar to that seen with atopic disease.1 When eosinophilia is marked, this association provides a clinically useful sign of a migratory worm infection. The prominent peripheral blood eosinophilia of tissue migratory worm infections contrasts with the lack of eosinophilia seen in people in whom the worm infection is confined to the gut lumen or in whom the infection is due to another agent (e.g., viruses or bacteria). It should be noted that patients who lack cellular immune function are often unable to produce this characteristic sign. Corticosteroids may also lyse eosinophils and complicate diagnosis.
Although it has long been thought that eosinophils play a vital role in the host defense against helminths, our understanding of their role has become much more sophisticated in recent years.1,2 Although eosinophils – along with antibodies or complement – kill the larval forms of several helminths in vitro, not all animal and human studies confirm the importance of eosinophils in host protection.1,3,4 Moreover, eosinophils may actually contribute to the pathogenesis and tissue destruction seen with certain helminthic infections.2 Currently, it is thought that immunity to the helminths does not rest on any single cell type, but rather a coordinated effort from multiple immune pathways. Although the adaptive immune system plays a vital role in coordinating this attack via sensitized lymphocytes, much of our immunity to parasites rests with innate pathways of host defense.2,5 It has also become increasingly recognized that sterilizing immunity to helminths is rarely achieved, likely due to their sophisticated mechanisms of immune evasion and the high cost of fully eradicating these large, multicellular organisms Rather, most of the time, humans and helminths exist in a state of evolutionary-derived tolerance.
HOST–PARASITE RELATIONSHIP IN PULMONARY HELMINTHIASES
Human disease caused by pulmonary helminthiases results from various factors. Many helminths can reside in human lungs during one or more of their parasitic stages. However, the stage of the life cycle that causes human pulmonary disease varies—for example, larvae of nematodes, eggs of schistosomes, and adult worms in paragonimiasis or cestode infections. The multiplicity and complex structure of these etiologic agents lead to a heterogeneous set of responses, both immunologic and nonimmunologic. Moreover, disease may result from the mechanical presence of worms (i.e., space-occupying lesions) and the associated inflammatory response, as a byproduct of the host immune response, or both. For example, in echinococcosis, hydatid cysts displace lung tissue, but in pulmonary schistosomiasis, the vascular obstructive lesions are predominantly the outcome of the delayed-hypersensitivity response of the host causing granuloma formation. Therefore, the understanding of the host–parasite relationship in pulmonary helminthiasis is based on an appreciation of the heterogeneity of these etiologic agents and the corresponding host responses.
The immune responses of the host often feature prominently in shaping the pathologic consequences of helminthic infection of the lungs. In experimental animals, the degree of tissue injury and host responsiveness to several helminthiases has been shown to be regulated by modulatory antibody, cellular, and cytokine responses. Whether humans acquire resistance to helminthic infection and whether resistance can be induced has not been fully resolved. Resistance against several helminths occurs in experimental and wild animals after primary infection and can be induced by defined antigens.6 It can also be seen in individuals who avoid certain parasitic infections, such as lymphatic filariasis, despite living in worm endemic areas.7
An additional and biologically relevant factor concerning helminthic infections and their role in the etiology of human disease is the intensity of infection. Since most worms that infect humans cannot increase their population without additional exposure to the infective stages, the worm load largely determines the degree of pathologic sequelae. For example, the number of schistosome eggs reaching the pulmonary circulation is an essential determinant of the severity of the induced disease. Although the number of eggs reaching the lungs may be influenced by several factors, the most important determinant is the number of adult worms in the infected person.
APPROACH TO THE PATIENT WITH HELMINTHIC INFECTION OF THE LUNGS
The major symptoms and signs of pulmonary disease are similar across different etiologic agents—infectious and noninfectious alike. Certain clues in the history are necessary to trigger the consideration of a helminthic infection. The geographic distributions of the major helminthic infections are roughly known. Therefore, a history that the patient has lived overseas or in certain parts of the United States is helpful in alerting the examiner to the possibility of a helminthic infection. Although helminthic infections are generally more common in temperate and hot areas of the world, certain parasites may be acquired in the United States and other colder areas. Other infections are acquired from specific animal exposures or occupations, such as E. granulosus and exposure to dogs and sheep. Therefore, infection with E. granulosus is more likely in sheep-raising countries and in certain sheep-raising areas in the United States.
Eosinophilia in peripheral blood, sputum, or pulmonary tissue is a helpful clue in directing the diagnostic workup. Although increased eosinophil counts do occur in several other pulmonary diseases, the close association with helminthic infections necessitates appropriate diagnostic procedures to determine whether a worm is implicated. However, eosinophilia may not be present for all individuals. Knowledge of the immunologic status of the patient can be valuable in suggesting helminthic infection in the absence of eosinophilia—for example, the hyperinfection syndrome caused by S. stercoralis.
Definitive diagnosis of pulmonary helminthic infections requires isolation and identification of the parasite that routine examination of appropriate specimens may miss. Laboratory personnel should always be alerted when the examiner is considering the possibility of a worm infection so that proper samples can be obtained and preserved for special examinations. Serologic testing for helminths is particularly useful in nonendemic settings. It should be considered an adjunct to isolation of the pathogen and an important diagnostic procedure.
DISEASES DUE TO NEMATODES (ROUNDWORMS)
Among the most important helminthic infections of humans are the nematodes. The biology, clinical manifestations, and management of nematode infections are discussed below.
 ASCARIASIS, HOOKWORMS, AND STRONGYLOIDIASIS
ASCARIASIS, HOOKWORMS, AND STRONGYLOIDIASIS
Human infections with Ascaris lumbricoides, the hookworms Ancylostoma duodenale and Necator americanus, and S. stercoralis are among the most prevalent helminthiases worldwide. Transmission is seen in the southeastern United States.
Etiology
Human ascariasis (Fig. 137-1) results from ingestion of embryonated A. lumbricoides eggs that are contained in feces-contaminated soil. Ingestion of contaminated fruits and vegetables that have not been properly washed is the most frequent transmission vehicle. Ascaris eggs hatch in the gastrointestinal tract, producing larvae that penetrate the gut wall and migrate via venous blood and the right side of the heart to the lungs. Hookworms (A. duodenale and N. americanus) and S. stercoralis infect humans when infective larvae in the soil penetrate intact skin. The larvae then travel via the bloodstream to the lungs (Table 137-1). Next, they migrate via the pulmonary capillaries into the alveolar spaces. Once in the alveolar spaces, they ascend in the trachea and, ultimately, are swallowed to begin their final route to habitation in the small intestine. Here, the adults lay eggs that are either directly passed in the stool (A. lumbricoides, A. duodenale, and N. americanus) or hatch into larvae within the intestinal tract (S. stercoralis) and then passed into the stool. Although larvae are sometimes found in the sputum of infected persons, more often examination of the stool yields the diagnosis. Passage to the outside environment, where the noninfectious stool forms develop into forms infective for humans, completes their life cycle.
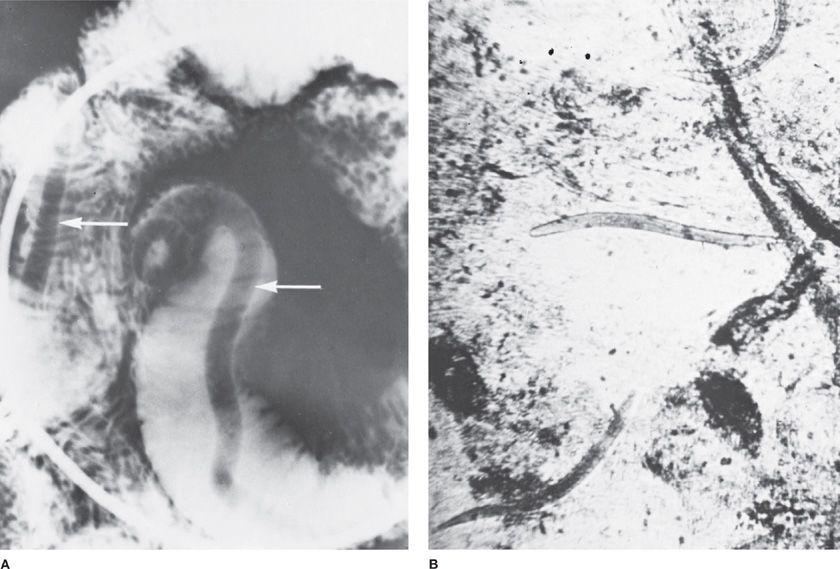
Figure 137-1 Nematodes. A. Ascariasis. Barium in the small intestine outlines two Ascaris worms (arrows). B. Strongyloides stercoralis. Rhabditiform larvae. (Used with permission of Dr. Stanley H. Abadie.)
Pathogenesis and Pathology
In nematode infections, the most prominent pulmonary pathologic changes occur in persons with ascariasis or the hyperinfection syndrome of strongyloidiasis. Pulmonary symptoms from Ascaris may occur 1 to 3 weeks after primary infection. Portions of larvae are seen in the pulmonary parenchyma, surrounded by a patchy infiltrate of neutrophils and eosinophils. The alveoli contain a serous exudate and the production of bronchial mucus is increased. Later, migrating larvae are destroyed within aggregates of eosinophils. The intensity of the reaction depends on the number of parasite larvae and previous sensitization. In areas in which transmission of Ascaris eggs occurs seasonally, pulmonary reactions are usually more frequent during these periods.8
In immunocompetent subjects, pulmonary disease caused by hookworms or S. stercoralis is minimal as the worm burden is usually low. It should be noted that infection with Strongyloides is lifelong. In nonimmunosuppressed subjects, the majority of the rhabditiform larvae found in the stool have to go to the outside world to transform into the infective filariform larvae. Only a few infective filariform larvae develop in the gut and penetrate the intestinal mucosa to restart the developmental cycle (autoinfection).
The sequence of events in immunosuppressed patients indicates that a change has occurred in the reproductive cycle of the parasite. In immunosuppressed patients, adult females may replicate by pathogenesis and the change to infective filariform larvae more frequently occurs within the gut lumen leading to a sharp increase in worm burden.9 The infective filariform larvae penetrate the intestinal mucosa resulting in massive invasion of almost every organ, including the lungs. Consequently, life-threatening infection with S. stercoralis results from the premature development of large numbers of filariform larvae in immunocompromised persons that invade across the gut wall or perianal skin, carrying intestinal bacteria into the peritoneum and bloodstream.9 Tissue migration of the worms occurs through most body organs, including the lungs. Initially, the pulmonary lesions resemble those of Ascaris pneumonia. In some patients, bronchopneumonia and lung abscesses develop while the lungs of fatal cases show intra-alveolar hemorrhages and inflammatory changes.10
Clinical Features
The major clinical manifestations caused by infection of the lungs with the larval forms of intestinal nematodes resemble those described by Loeffler;11 these manifestations occur typically in patients with Ascaris pneumonia, but rarely have been reported in patients with hookworm pneumonia.8,12 Symptoms include persistent, irritating, and nonproductive cough, substernal pain, and – in the severely ill – hemoptysis and dyspnea. Eosinophilia is the most consistent laboratory finding. Radiographic signs – for example, patchy or miliary infiltrate – are sometimes seen. Ascaris suum (pig roundworm) may cause a similar pulmonary process, although it does not exhibit all of the same developmental stages as A. lumbricoides since humans are only accidental hosts.13
The onset of the Loeffler-like syndrome caused by intestinal nematodes usually occurs 1 to 3 weeks after infection, coincident with larval migration from the pulmonary circulation to the alveoli. This timing was illustrated in a report of a group of students exposed to eggs of A. suum.14 Typical pulmonary symptoms occurred 10 to 15 days later, and some of the students developed marked respiratory failure. In locations in which transmission of ascariasis is cyclic because of environmental factors, pneumonitis occurs seasonally.8 Mild symptoms are occasionally encountered in persons with hookworm infection or in immunocompetent subjects who have strongyloidiasis.
The most clinically significant pulmonary syndrome induced by intestinal nematodes is that caused by hyperinfection with S. stercoralis (Fig. 137-2). As a rule, the syndrome occurs in patients with compromised cell-mediated immunity because it is a consequence of a high worm burden. However, it is occasionally encountered in normal persons. Immunosuppression may be the result of neoplastic diseases, including lymphomas and leukemias, or nonmalignant conditions that are being treated with corticosteroids – for example, organ transplantation. However, the autoinfection cycle means that disease due to S. stercoralis, including hyperinfection, may develop as late as 75 years after initial exposure.15
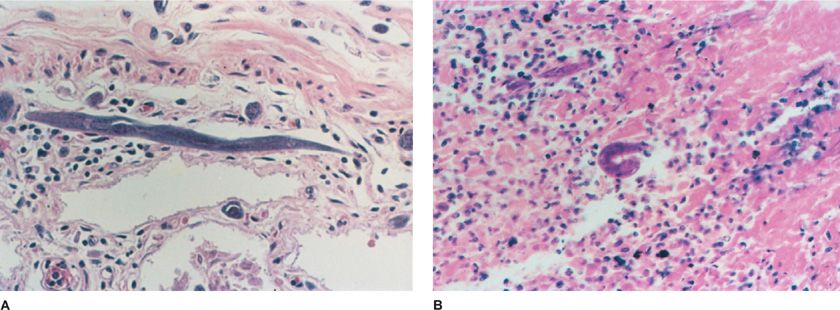
Figure 137-2 Strongyloidiasis. A. A 55-year-old man with chronic lymphocytic leukemia who presented with abdominal discomfort and weight loss. The patient developed progressive pulmonary congestion and edema with dyspnea, fever to 103°F, and progressive hypotension before death. Blood cultures revealed Escherichia coli. Histologic section of colon at autopsy shows adult S. stercoralis in wall. B. A 24-year-old man with AIDS who developed diarrhea, weight loss, and, finally, shock with E. coli bacteremia and strongyloidiasis. The larval form is shown in the jejunum. (Used with permission of Dr. Jay A. Fishman.)
The major pulmonary clinical features include asthma, pulmonary opacities, which include cavitation, consolidation, and diffuse patchy infiltrates.10 Usually, widespread dissemination of the nematode is accompanied by secondary infection caused by gram-negative bacteria carried along with S. stercoralis from the gut. Consequently, gram-negative meningitis and sepsis may also be prominent features of this syndrome. Eosinophilia is often absent in patients with the S. stercoralis hyperinfection syndrome, due to either defective cell-mediated immunity or the use of corticosteroids in many patients. The S. stercoralis hyperinfection syndrome is often fatal; mortality occurs in up to 87% of people with disseminated infections.16
Management
The diagnosis of infection with intestinal nematodes that causes a Loeffler-like syndrome may be difficult. Only occasionally is the search for parasite larvae in sputum rewarding. Stool examination is negative at this point because the adults have not yet reached the small intestine and begun producing eggs. Not infrequently, definitive diagnosis is delayed for weeks until the adult worms mature in the small intestine. At this stage, fecal examination discloses the characteristic eggs of hookworms or Ascaris or the larvae of S. stercoralis. The management of patients with the pulmonary manifestations of these parasitic worms is nonspecific and symptomatic. Reduction of exposure in areas in which transmission of ascariasis is seasonal decreases the prevalence and severity of clinical presentations. Specific antihelminthic therapy is ineffective during the pulmonary stage but can cure the infection once the parasites reach maturity in the small intestine.
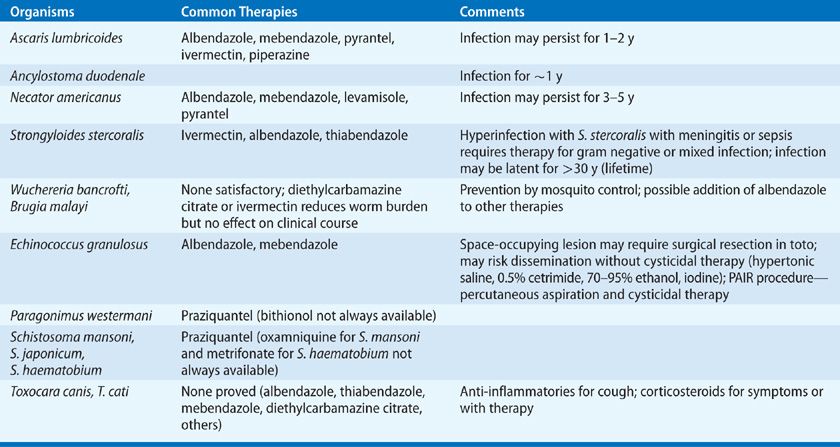
Albendazole, 400 mg orally once, or mebendazole, 100 mg per day for 2 to 3 days (or 500 mg orally once) are the drugs of choice for treating ascariasis and hookworms (Table 137-2). Ivermectin, 200 μg/kg per day for 2 days (longer for hyperinfection syndrome) is recommended for strongyloidiasis.17 This treatment may be repeated at 2 weeks (the duration of one autoinfection cycle) to ensure eradication is complete. Albendazole, 400 mg orally daily for 2 days may be used as an alternative, but it is less effective.17,18
TABLE 137-2 Therapies of Pulmonary Diseases Produced by Worms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree