Heart Disease and Pregnancy: Introduction
Heart Disease Issues Unique to Pregnancy
The health of the developing fetus is predominantly determined by the health of the mother. When treating heart disease, the health of the fetus should be considered, but the safety of the mother is the highest priority. Ideally, treatment of the mother with drugs, diagnostic studies, or surgery should be avoided unless required for maternal safety.
Heart disease is the second most common cause of maternal death in Western countries (suicide is first).1 Sometimes the risk is sufficient to recommend avoidance or interruption of pregnancy (Table 97–1).
| Advise avoidance or interruption of pregnancy |
| Pulmonary hypertension |
| Dilated cardiomyopathy with congestive failure |
| Marfan syndrome with dilated aortic root |
| Cyanotic congenital heart disease |
| Pregnancy counseling and close clinical follow-up required |
| Prosthetic valve |
| Coarctation of the aorta |
| Marfan syndrome |
| Dilated cardiomyopathy in asymptomatic women |
| Obstructive lesions |
The maternal commitment to the fetus is exceptional, but if the mother requires a redistribution of blood flow for her own safety, blood is preferentially diverted away from the uterus. This subjects the fetus to an insufficient supply of oxygen and nutrients and ineffective removal of metabolic waste and heat. Uterine blood flow can already be compromised in a woman with heart disease, increasing the possibility of inadequate uterine perfusion. Treatment of maternal heart disease can also jeopardize the fetus. Diagnostic studies, drugs, or surgery may increase fetal loss, result in teratogenicity, or alter fetal growth.
The health of a newborn infant is a concern when the mother has heart disease. This fragility can be caused by a marginal uterine blood flow during pregnancy or by lingering effects of the medications used to treat the mother. Additionally, there is an increased incidence of congenital heart disease among the live-born infants of parents with congenital heart disease. Early infant nourishment may be jeopardized if maternal heart disease is severe enough to interfere with breastfeeding. Even if the mother is capable of breastfeeding, cardiovascular medications can be transmitted to the infant in the breast milk. Finally, the infant is at risk of losing a parent because life expectancy with many forms of heart disease is significantly reduced.
Although new information has been acquired about hearts that have been altered by surgery (or a catheter), there is still much that remains unknown, particularly as it relates to pregnancy. It is best not to consider a previous lesion to have been mechanically corrected because there is always some residual disease.
A Warning
The combination of heart disease with pregnancy exceeds the expertise of most care providers and, in fact, exceeds the capabilities of any single provider. When possible, care before, during, and after pregnancy is best given by an experienced team that includes counselors, primary care providers, obstetricians, cardiologists, anesthesiologists, and pediatricians. Any woman contemplating pregnancy should be educated by experienced providers before conception.
Clinical Considerations
All of the advantages and disadvantages of each method of birth control apply to women with heart disease.2,3 Any can be considered, but potential fluid retention with depoprogesterone should guide its use. Although thromboemboli are of concern in many forms of heart disease, use of drugs with estrogen is safe if the estradiol content is less than 35 μg per tablet and the woman is a nonsmoker.
Antenatal care should include a discussion of the vulnerability issues explored above. The patient should be told which medications to avoid during pregnancy. Warfarin, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs) should be stopped (see discussion of drugs below). Any needed diagnostic tests or interventions should be performed before risk to the fetus becomes a factor.
Organ development in the fetus begins at 3 weeks, before some women even know they are pregnant. As soon as pregnancy is confirmed, drug use should be assessed, again avoiding warfarin, ACE inhibitors, and ARBs. Aspirin should be started in cyanotic patients. If not already done, referral to an appropriate center of expertise with heart disease and pregnancy should begin. Parent apprehension may be high, and education and support are helpful. Issues to address include warning symptoms, need for scheduled imaging, optimal site for delivery, and type of delivery.
The expected hemodynamic changes associated with pregnancy reach their peak near the 20th week. Women should be advised of the likely sensation of dyspnea. An obstetrician should monitor fetal growth and determine the need for fetal echocardiography.
If a pregnancy is at the 24th week or beyond and the mother develops a life-threatening situation such as uncontrollable pulmonary edema or a situation requiring emergency surgery, a cesarean section delivery should be considered.
Labor and delivery is a time of great demands on the cardiovascular system, and management must be optimized. Vaginal delivery is optimal in most patients with heart disease.4 However, if the second stage of labor is excessively painful or prolonged, the obstetricians should plan on assisted delivery (with forceps or vacuum suction) to shorten the second stage and should consider an assisted delivery depending on the severity of the mother’s heart disease. Induced labor or cesarean section should be reserved for obstetrical indications or worsening cardiovascular function. Exceptions to this include patients with extremely high-risk heart disease, including Eisenmenger syndrome and Marfan syndrome with aortic root dilatation, in whom an appropriately planned early delivery can be performed when the fetus is adequately mature. Oxytocin should be avoided because of potential hypotension.
In most cases, lumbar epidural anesthesia using low-dose techniques for cardiostability with a pudendal nerve block to minimize pain is effective and least likely to result in hemodynamic compromise5 and should be favored over general anesthesia. Antibiotic prophylaxis against bacterial endocarditis at the time of labor and delivery is practiced by most experienced centers. If it is used, new guidelines suggest it should be limited to women with previous endocarditis, a prosthetic valve, complex cyanotic heart disease, or a cardiac transplant.6
Successful delivery does not mean the mother is out of danger; a large proportion of maternal deaths occur more than 1 week after delivery.7,8 Hemodynamic and electrocardiographic (ECG) monitoring should be continued for 48 to 72 hours in those with severe abnormalities (eg, pulmonary hypertension, cyanotic lesions, severe obstructive lesions, or a severe cardiomyopathy). Important changes in clotting factors normally prevent excessive uterine bleeding, but these changes may disrupt the fragile thrombostasis in cyanotic patients. Warfarin can be reinstated carefully after delivery when necessary.
Cardiovascular Adjustments during a Normal Pregnancy
Maternal adaptation to pregnancy includes remarkable cardiovascular changes. These explain in part why some cardiac abnormalities are poorly tolerated during pregnancy (see Table 97–1).
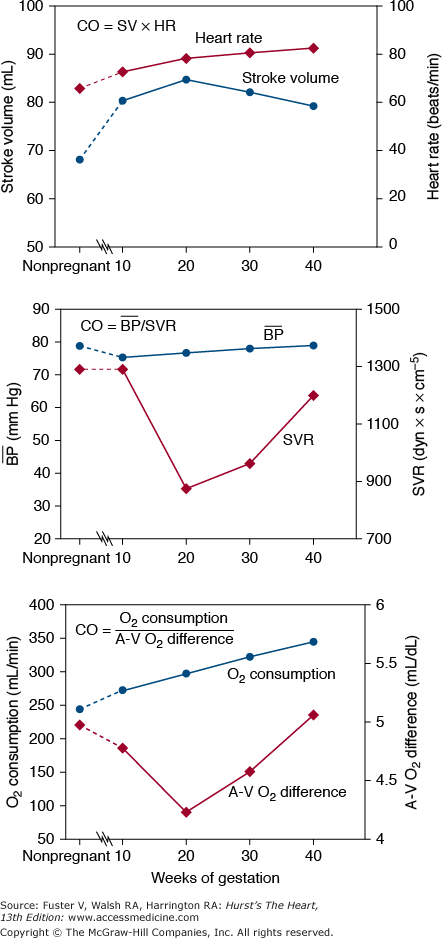
Resting cardiac output (CO) increases by more than 40% during pregnancy, reaching its highest levels by the 20th week (Fig. 97–1). Its early increase is caused mainly by an increase in stroke volume,9-12 with heart rate increasing gradually throughout pregnancy (Fig. 97–2). In the third trimester, CO is significantly affected by body position (see Fig. 97–1) because the enlarged uterus reduces venous return from the lower extremities.9-14 Compared with measurements made when the woman is in the left lateral position near term, CO is lower by an average of 0.6 L/min when a woman is supine and by 1.2 L/min when she assumes the upright position.15 In general, this results in few or no symptoms, but in some women, maintenance of the supine position may result in symptomatic hypotension, particularly when collateral vessels are not well developed.14 Symptoms of this supine hypotensive syndrome of pregnancy can be corrected by having the woman turn onto her side.
Figure 97–2.
The cardiac output (CO) can be determined from other parameters in at least three ways: CO = Heart rate (HR) × Stroke volume (SV); CO = Mean arterial pressure (blood pressure) − Right atrial (RA) pressure/Systemic vascular resistance (SVR); CO = Oxygen (O2) consumption/arteriovenous (AV) O2 difference. The expected values for these parameters measured in the supine position during pregnancy are based on information acquired from many studies.9-13
Blood pressure decreases slightly in early pregnancy. Systemic vascular resistance decreases until the 20th week and then gradually increases through the remainder of pregnancy (see Fig. 97–2). The mother’s oxygen consumption (which includes that of her fetus) increases by 20% within the first 20 weeks of pregnancy and increases steadily to a level that is approximately 30% above the nonpregnant level at the time of delivery.12 This increase is caused by both the metabolic needs of the fetus and the increased metabolic needs of the mother. These changes are better tolerated in patients with volume overload lesions (valvular regurgitation or shunts) than in patients with fixed output (obstructive valves, coarctation, or pulmonary hypertension).
At the beginning of labor, CO measured in the supine position increases to more than 7 L/min. This increases to more than 9 L/min with each uterine contraction because of extrusion of approximately 500 mL of blood into the central venous system and because of an increase in heart rate. Administration of epidural anesthesia reduces this CO to approximately 8 L/min, and the use of general anesthesia reduces it still further. After delivery, the CO briefly approaches 10 L/min15 (7-8 L/min with cesarean section)16; it then decreases rapidly to near-normal, nonpregnant values within a few weeks after delivery. A slight elevation in CO may persist for as long as 1 year.17 The increase in maternal CO in women with twins or triplets is only slightly greater than that in women with single pregnancies.15,18
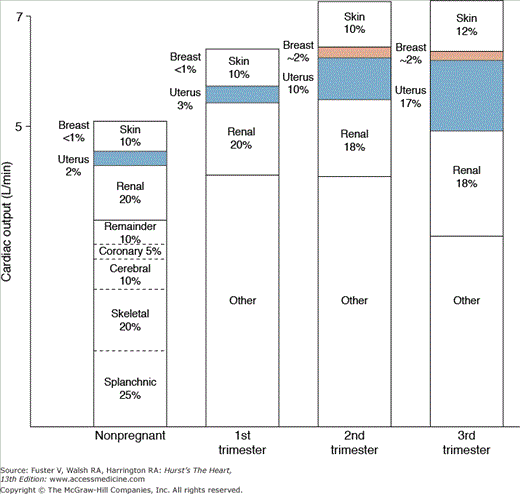
Pregnancy results in a redistribution of blood flow (Fig. 97–3). In nonpregnant women, uterine blood flow is approximately 100 mL/min (2% of the CO); it increases to approximately 1200 mL/min at term, a value approaching the mother’s blood flow to her own kidneys.19,20 During pregnancy, uterine blood vessels are maximally dilated; flow may increase, but this must result from increased maternal arterial. Excitement, heat, anxiety,21 exercise, and a decrease in venous return all decrease uterine blood flow. Vasoconstriction caused by endogenous catecholamines, vasoconstrictive drugs, maternal mechanical pulmonary ventilation, and some anesthetics, as well as that associated with preeclampsia and eclampsia, may decrease perfusion of the uterus.
Pregnancy changes the hemodynamic response to exercise.22 For any given level of exercise in the sitting position, the CO is greater than in nonpregnant women, and maximum CO is reached at lower exercise levels. During pregnancy, expected effects of conditioning or training on stroke volume are not seen, possibly because of uterine compression of the inferior vena cava or the increased venous capacitance.23
Exercise during pregnancy is not clearly any more dangerous or beneficial to a woman with heart disease than when she is not pregnant. The fetus is affected. In animal models, maternal exercise has been associated with a decrease in uterine blood flow. In humans, the type of exercise affects maternal hemodynamics and uterine perfusion.24-26 Additionally, regular aerobic endurance exercise during pregnancy has been associated with a reduction in birth weight. It is not clear if this is detrimental.27
Infants born to mothers who work in a standing position can be abnormally small at birth.28 Although the long-term effects of this are not clear, the implications in relation to exercise and work in the upright position are likely greater for women with heart disease.29-31 There is enthusiasm for recreational exercise in the United States. Although there is an insufficient amount of data to suggest that healthy pregnant women should avoid recreational exercise, an argument can be made for advising women with heart disease to keep the exercise level below that which causes symptoms.32
The mechanisms involving this adaptation to pregnancy are not totally understood. They can in part be caused by volume change. Total body water increases steadily throughout pregnancy by 6 to 8 L (most is extracellular).33 Sodium retention results in an excess accumulation of 500 to 900 mEq by the time of delivery. As early as 6 weeks after conception, plasma volume increases, approaching its maximum of 1.5 to 2 times normal by the second trimester, where it stays throughout the pregnancy.34 The red blood cell mass also increases but not to the same degree. Thus, the hematocrit decreases, although rarely to less than 30%.
Intrinsic cardiac changes can also explain some of the hemodynamic changes.35-37 The stroke volume increases by approximately 25%. The ejection fraction does not change. Because the increases in left ventricular (LV) end-diastolic and systolic volumes are small and not adequate to explain the constant ejection fraction, the heart must become reconfigured as well. Some evidence suggests that in some disease states, this remodeling persists after pregnancy.38
Vascular alterations also contribute to the hemodynamic changes of pregnancy. Arterial compliance is increased.39,40 Venous capacitance increases as well, although there is an increase in venous vascular tone.41 These changes are advantageous in maintaining the hemodynamics of a normal pregnancy. There can be disadvantages as well; vascular accidents, when they occur in women, frequently do so during pregnancy.42,43 Additionally, the venous changes can explain, in part, the increase in thromboemboli during pregnancy.44 The ultimate cause of these recognized changes is uncertain. Complex interactions of the renin-angiotensin-aldosterone system,45 prostaglandins, nitric oxide, and atrial and brain natriuretic factors46 contribute to the fluid and sodium changes. Currently, the effects of the increased level of circulating reproductive hormones seem to satisfactorily explain the vascular and myocardial changes.
Diagnosis of Heart Disease
In a normal pregnancy, symptoms (dyspnea, fatigue) and signs (a third heart sound [S3], pedal edema) mimic those of heart disease, making diagnosis difficult. Symptoms that should alert a caregiver to the possibility of heart disease include limiting dyspnea or orthopnea, hemoptysis, syncope with exertion, or chest pain clearly related to effort. On examination, cyanosis or clubbing, a loud systolic murmur (grade 3 or louder) or any diastolic murmur suggest heart disease. Venous hums or internal mammary flow sounds (the mammary souffle), which have diastolic components, are findings during a normal pregnancy.
Echocardiography is safe (no known risk to the mother or fetus) and is so diagnostically useful that overuse, expense, and potential misinterpretation are the only significant concerns. Chamber dimensions and velocity measurements need to be interpreted considering the hemodynamic changes outlined above.
Electrocardiography is safe, although pregnancy makes interpretation of ST-T wave variations even more difficult than usual. Inferior ST-segment depression is common enough to possibly be the result of a normal pregnancy. There is a leftward shift of the QRS axis during pregnancy, but true axis deviation (−30 degrees) implies heart disease.
Cardiac magnetic resonance imaging (MRI) is also generally safe during pregnancy, although administration of gadolinium is contraindicated47
All radiation procedures, including computed tomography (CT), nuclear scans, and catheterization, should be avoided unless absolutely necessary. This problem relates to long-term maternal health48 but particularly relates to fetal vulnerability, with an increased risk of abnormal fetal organogenesis or of a subsequent malignancy in the child, particularly leukemia. Although estimated exposure to the fetus from a chest radiograph (10-1400 microgray [μGy]) or radionuclide scan is low (400 μGy), even these should be avoided unless necessary. If a study is required, it should be delayed to as late in pregnancy as possible, the radiation dose should be kept to a minimum, and shielding of the fetus should be optimized.
Cardiovascular Drugs and Pregnancy
Nearly all cardiac drugs cross the placenta and are secreted in breast milk. Because information about the use of any drug is incomplete, it is best to avoid drug use, but if required for maternal safety, drugs should not be withheld. Although limited because of incomplete data (and with a potential update in process), a US Food and Drug Administration (FDA) classification of drugs as they relate to fetal safety provides broad guidance for drug use during pregnancy (Table 97–2).
| FDA Pregnancy Category | |
|---|---|
| A | Human and animal studies have not shown fetal risk. Use for maternal safety and symptoms appropriate.a |
| B | No adequate human studies. Animal studies have not shown fetal risk. Use for maternal safety or severe symptoms appropriate. |
| C | No adequate studies in humans. Teratogenicity has been shown in animal studies. Use for maternal safety may be justified. |
| D | Demonstrated fetal risk in human (and animal) studies. Maternal safety would seem to be the only justification for use during pregnancy. |
| X | Demonstrated fetal risk in human and animal studies of sufficient severity to recommend that drug not be used during pregnancy. Only extreme maternal safety issues justify use. |
Diuretics can and should be used for treatment of congestive heart failure that is uncontrolled by sodium restriction and for the treatment of hypertension.49,50 They should not be used for prophylaxis against toxemia or for treatment of pedal edema.
The indications for the use of digitalis are not changed by pregnancy. The same dose of digoxin in general will yield lower maternal serum levels during pregnancy than in the nonpregnant state. Fetal serum levels approximate those in the mother. The drug is often given to mothers to treat fetal arrhythmia.
When intravenous (IV) inotropic or vasopressor agents are required, the standard agents (dopamine, dobutamine, and norepinephrine) can be used, but the fetus is jeopardized because all such agents increase resistance to uterine blood flow and can stimulate uterine contractions. Ephedrine is an appropriate initial vasopressor drug because, at least in animal models, it does not adversely affect uterine blood flow.
There is little information about the efficacy or safety of the phosphodiesterase inhibitors (amrinone, milrinone) in pregnancy.
Observations that β-blockers can decrease umbilical blood flow, initiate premature labor, and result in a small and infarcted placenta with the potential for low-birth-weight infants have led to concerns about their use. However, these drugs have been used in a large number of pregnant women without adverse effects. Their use for the usual clinical indications is reasonable.51 Metoprolol (FDA class B) is favored over atenolol (FDA class C). If these agents are used during pregnancy, it is appropriate to monitor fetal and newborn infant heart rate, blood sugar, and respiratory status.
Experience with the α-blocking agents phenoxybenzamine and phentolamine is sparse. Clonidine, prazosin, and labetalol, with their mixed α- and β-blocking effects, have been used for the treatment of hypertension during pregnancy without clear detrimental effects.50
The dihydropyridine agents are effective antihypertensive and afterload-reducing agents that have been used without any adverse effect on fetuses or newborn infants. If a nondihydropyridine agent is required, verapamil (FDA class B) is favored over diltiazem (FDA class C). The calcium channel blockers cause relaxation of the uterus; nifedipine has been used for this purpose.
When atrioventricular (AV) node blockade is required during pregnancy, β-blockers, calcium blockers, adenosine, or digoxin can be used.52 As a general rule, it is preferable to avoid the standard antiarrhythmic drugs in any patient. This is true during pregnancy as well. When such drugs are essential for the treatment of recurrent arrhythmias or for maternal safety, they should be used. If IV drug therapy is required, lidocaine or procainamide provide reasonable first-line therapy; there is scant reported experience with IV amiodarone or ibutilide.
If oral antiarrhythmic therapy is necessary, flecainide and sotalol (FDA class C) are as likely to be as effective as other drugs, and fetal safety is reasonable; they are often given to the mother to treat the fetus.53 Quinidine has also been used frequently without clear adverse fetal effects. Information about procainamide, disopyramide, mexiletine, and dofetilide (FDA class C) is sparse.54 The early available information concerning amiodarone (FDA class X) indicates a 10% chance of fetal thyroid abnormalities and an increased likelihood of fetal loss and deformity.55 Thus, it should be avoided unless its use is essential for maternal or fetal safety. There is no information for dronedarone.
When needed for a true hypertensive crisis or emergency afterload and preload reduction, nitroprusside is the vasodilator drug of choice. It is highly effective, works instantly, and is easily titrated, and its effects dissipate immediately when the drug is stopped. A concern that its metabolite, cyanide, can be detected in the fetus has not been a demonstrated significant problem in humans.56,57 Concern about toxicity is a reason to replace it with alternative drugs such as parenteral hydralazine, nitroglycerin, or labetalol when the most acute situation is made more stable.
Chronic afterload reduction to treat hypertension, aortic or mitral regurgitation, or ventricular dysfunction during pregnancy has been achieved with the calcium blocking drugs hydralazine and methyldopa. Adverse fetal effects have not been reported.
The ACE inhibitors (FDA class X) increase the risk of major congenital abnormality and are contraindicated in pregnancy.58-60 A series of case reports of similar problems with the ARBs suggest that their use should be avoided until more data are available.61,62
Prostaglandins (epoprostenol and iloprost), phosphodiesterase type 5 inhibitors (sildenafil), and nitric oxide (FDA classes B and C) have been used successfully as vasodilators to treat pulmonary hypertension with no reported adverse maternal or fetal effects. 63,64 The endothelin receptor blocker bosentan (FDA class X) should not be used because of concerns of teratogenicity.
Warfarin is contraindicated during the first 3 months (particularly weeks 7-12) of pregnancy because it crosses the placenta and is associated with a 1% to 25% incidence of malformations that comprise the warfarin embryopathy syndrome (facial abnormalities, optic atrophy, digital abnormalities, epithelial changes, and mental impairment).65,66 The syndrome can be dose related, with one study suggesting that it occurs only with doses greater than 5 mg/d.67 The use of warfarin at any time during pregnancy increases the risk of fetal and maternal bleeding. Warfarin is an FDA class X during the first trimester and is best considered a class B drug for the remainder of pregnancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree