Heart and Lung Transplantation in Children
Stephanie Fuller
Thomas L. Spray
Clinical heart-lung transplantation began with Cooley in the late 1960s when the first heart-lung transplant was performed in an infant with atrioventricular canal defect and pulmonary hypertension. The child died of pulmonary insufficiency 14 hours after the operation. The first clinical success was not achieved until 1981 when Reitz performed a heart-lung transplant in a 45-year-old patient with pulmonary hypertension. As success was achieved in adult patients, heart-lung transplantation was performed in an increasing number of pediatric patients, from only a few in 1984 to as many as 40 in 1988. Successful clinical use of isolated lung transplantation in an adult by Cooper in 1984 established the possibility of lung transplantation alone for certain forms of cardiopulmonary disease. These techniques were ultimately extended to the pediatric population such that the use of heart-lung transplantation in children has gradually been restricted to children with irreparable cardiac defects associated with pulmonary disease while isolated lung transplantation (either single or bilateral) has become an accepted modality of therapy for children with pulmonary vascular disease or primary pulmonary disease with reparable cardiac defects. By 2012, approximately 1,200 children, the majority adolescent, have undergone single-lung or bilaterallung transplantation. The number of heart-lung transplantations in the pediatric population has declined significantly and is rare in the United States.
INDICATIONS FOR TRANSPLANT
General indications for heart-lung or lung transplantation in pediatric recipients are similar to those in adults. End-stage restrictive or obstructive parenchymal pulmonary disease or primary or secondary pulmonary vascular disease associated with correctable congenital heart defects can be considered for cardiac repair and lung transplantation if the cardiac repair is durable and not associated with significant inherent mortality. The presence of significant left ventricular dysfunction or cardiopulmonary defects that are uncorrectable are best treated by heart-lung transplantation.
Although general indications for lung transplantation are similar in adults and children, the types of pulmonary disease seen in children are different from those in adults. Obstructive pulmonary disease is extremely unusual in children as is pulmonary fibrotic disease. The great majority of lung transplants in children >5 years old are for cystic fibrosis. If present, fibrotic disease other than cystic fibrosis is often due to radiation fibrosis as a consequence of the treatment for leukemia and lymphoma. Younger children, under the age of 6, are transplanted for idiopathic pulmonary arterial disease and congenital heart disease with pulmonary venous stenosis. Neonates are only usually referred for significant surfactant abnormalities such as surfactant B or C deficiency for which there is no other treatment.
Children with cystic fibrosis represent the single largest group of pediatric patients who require lung or heart-lung transplantation. Most of these children are teenagers because the majority of cystic fibrosis patients survive to adulthood with intensive medical management and suffer from a slow decline. Indications for consideration of pulmonary transplantation in cystic fibrosis patients include increasing hospitalization for infection, progressive weight loss in older patients or lack of weight gain in younger patients despite nutritional supplementation, and increase in oxygen dependence or hypercarbia with gradual deterioration of pulmonary function. In general, a forced expiratory volume in 1 second (FEV1) of <30% of predicted values is a relative indication for transplantation.
Cardiopulmonary diseases requiring transplantation in infancy are rare but include congenital diaphragmatic hernia, surfactant protein deficiencies, pulmonary vein stenosis or veno-occlusive diseases, and primary pulmonary hypoplasia.
The indications for transplantation in children with pulmonary hypertension are somewhat subjective because of the poorly defined natural history of pulmonary hypertension, either primary or secondary, in this age group. Generally, indications for consideration include progressive exercise intolerance, the onset of syncope, hemoptysis, angina pectoris, and significant right ventricular failure. These symptoms are often correlated with hemodynamic abnormalities, with elevations of right atrial pressure to >8 mmHg with a decreased cardiac index and a total pulmonary vascular resistance of >20 Woods units/m2. When the product of right atrial mean pressure and pulmonary vascular resistance index is >360, poor survival is expected and transplantation is considered. Some children with significant pulmonary vascular disease may respond to vasodilators, including prostacyclin, and the use of chronic prostacyclin infusion may improve the stability of patients while they await suitable donor organs. The availability of increasingly novel therapies for pulmonary hypertension has decreased the need for lung transplantation in a significant number of patients. Most patients can now at least have significant palliation of their disease by medical management with transplantation reserved only for those patients who develop right heart failure or other major complications. In patients with Eisenmenger syndrome and a right-to-left cardiac shunt, the onset of severe polycythemia in association with hemoptysis, right ventricular failure, or progressive exercise intolerance may be considered a relative indication for transplantation.
A particularly difficult subgroup of patients comprises those with pulmonary vein stenosis and pulmonary veno-occlusive disease. These children are often extremely unstable with severe pulmonary hypertension. Because delivery of blood to the left ventricle is limited in these
patients, there has been a high incidence of death while awaiting organs, and therefore these children should be listed early in the course of their disease. It is important to recognize patients who have pulmonary vein stenosis or veno-occlusive disease that is not amenable to further surgical therapy. Despite the increased enthusiasm for the use of sutureless pulmonary vein repair in patients with pulmonary venous stenosis, there are no significant data that this approach has decreased the incidence of recurrent obstruction and in most of these patients the process appears to be a progressive disease. The lack of dilation of the pulmonary veins in the hilum of the lung in the presence of pulmonary vein stenosis is a marker that there are abnormalities of the veins that will not respond to surgical intervention. In addition, very small veins at the time of repair of total anomalous pulmonary venous return are associated with very poor long-term outcome and these patients may also be considered for transplantation. Patients who have stenosis of only one or two pulmonary veins or occlusion of one vein with other veins not obstructed yet in the presence of significant pulmonary hypertension are a very poor subset of patients for any surgical intervention. These patients who will not respond to surgical therapy should be considered for lung transplantation. Stenting of stenotic pulmonary veins may be palliative in some cases as a bridge to pulmonary or cardiopulmonary transplantation. Although it is possible to use chronic ventilation, nitric oxide therapy and even extracorporeal membrane oxygenation (ECMO) as a bridge to lung or cardiopulmonary transplantation in children, patients with pulmonary vein stenosis or pulmonary vascular disease are often unable to be adequately resuscitated with chest compressions while ECMO support is initiated because of the inability to get adequate blood flow to the left ventricle to provide cerebral blood flow during cardiac arrest. Heart-lung transplantation provides an option for patients who have uncorrectable congenital heart disease and pulmonary vascular disease. Typically, these patients become candidates once palliation is no longer successful, once exhibiting severe heart failure requiring inotropic support, or severe pulmonary hypertension requiring inhaled nitric oxide or infused prostaglandin.
patients, there has been a high incidence of death while awaiting organs, and therefore these children should be listed early in the course of their disease. It is important to recognize patients who have pulmonary vein stenosis or veno-occlusive disease that is not amenable to further surgical therapy. Despite the increased enthusiasm for the use of sutureless pulmonary vein repair in patients with pulmonary venous stenosis, there are no significant data that this approach has decreased the incidence of recurrent obstruction and in most of these patients the process appears to be a progressive disease. The lack of dilation of the pulmonary veins in the hilum of the lung in the presence of pulmonary vein stenosis is a marker that there are abnormalities of the veins that will not respond to surgical intervention. In addition, very small veins at the time of repair of total anomalous pulmonary venous return are associated with very poor long-term outcome and these patients may also be considered for transplantation. Patients who have stenosis of only one or two pulmonary veins or occlusion of one vein with other veins not obstructed yet in the presence of significant pulmonary hypertension are a very poor subset of patients for any surgical intervention. These patients who will not respond to surgical therapy should be considered for lung transplantation. Stenting of stenotic pulmonary veins may be palliative in some cases as a bridge to pulmonary or cardiopulmonary transplantation. Although it is possible to use chronic ventilation, nitric oxide therapy and even extracorporeal membrane oxygenation (ECMO) as a bridge to lung or cardiopulmonary transplantation in children, patients with pulmonary vein stenosis or pulmonary vascular disease are often unable to be adequately resuscitated with chest compressions while ECMO support is initiated because of the inability to get adequate blood flow to the left ventricle to provide cerebral blood flow during cardiac arrest. Heart-lung transplantation provides an option for patients who have uncorrectable congenital heart disease and pulmonary vascular disease. Typically, these patients become candidates once palliation is no longer successful, once exhibiting severe heart failure requiring inotropic support, or severe pulmonary hypertension requiring inhaled nitric oxide or infused prostaglandin.
Infectious complications are more common after cardiopulmonary and pulmonary transplantation than after solid-organ transplants. Of particular importance in pediatric recipients is the common occurrence of viral infections of the transplanted lungs. Children continue to be exposed to a wide range of viruses, including adenovirus and respiratory syncytial virus in addition to the influenza viruses. In addition, cytomegalovirus (CMV) infection is very common after pediatric lung transplantation because many children have not yet been exposed to CMV infection and active immunity is not present. An additional concern in children is the presence of Ebstein-Barr virus (EBV). There is a higher incidence of seronegativity for this viral pathogen in children than in adults, and EBV infection can be quite subtle in early childhood, making diagnosis difficult. A significantly increased risk of associated lymphoproliferative disease in patients who have sustained an EBV infection after transplant has been reported and may progress despite decreased immunosuppression in these patients.
CONTRAINDICATIONS TO TRANSPLANTATION
Contraindications to transplantation are malignancy, multisystem organ failure, and sepsis. Other contraindications are primarily mechanical. Patients with severe scoliosis or restrictive chest wall mechanics may have chronic hypoventilation even if normal lungs are implanted. Significant associated metabolic diseases including renal insufficiency or uncontrolled diabetes are relative contraindications to transplantation, and patients with portal hypertension and biliary cirrhosis may also be considered poor candidates for cardiopulmonary transplantation alone. Patients who have had multiple prior surgical procedures with involvement of the pleural spaces require more complicated operations for implantation of donor organs. This is a particular problem in patients with chronic cyanosis, who may have multiple and extensive collateral vessels in the adhesions from previous surgeries that may be difficult or impossible to control at the time of transplant. Severe and even fatal bleeding complications have been noted in these patients in our series. High-dose steroid dependence is a relative contraindication to transplantation because of poor wound healing or sepsis. However, moderate doses of steroids have not been considered a contraindication in our experience. A long-standing history of noncompliance with medical interventions by the patient or family may be considered a relative contraindication to undertaking a procedure of such magnitude. Uncontrolled collagen vascular disease is also a contraindication to consideration for transplantation.
SELECTION OF OPERATIVE PROCEDURE
Historically, the majority of transplantations in children have been by the heart-lung en bloc technique. Whereas it is apparent that comparable results can be obtained with heart-lung or lung transplantation in many children, the need to maximize the availability of scarce donor organs and use the heart for other patients has produced a gradual decrease in the use of combined heart-lung transplantation for primary pulmonary diseases. Most heart-lung transplantation is now reserved for patients with uncorrectable congenital heart defects associated with severe pulmonary vascular disease. Heterotopic heart transplantation may be used in some children with elevated pulmonary resistance in whom improvement in pulmonary resistance can be anticipated with improvement in cardiac output. We use heart-lung transplantation for only those children with congenital heart disease who have a poor chance of long-term correction or those children with severe left or biventricular dysfunction. Pulmonary transplantation with preservation of the native heart is preferred in children with primary or secondary pulmonary vascular disease associated with normal ventricular function and a relatively simple or correctable congenital heart defect. Even children with severe right ventricular dysfunction are considered candidates for pulmonary transplantation alone if the right ventricular ejection fraction is >10% and tricuspid valve insufficiency is graded less than severe. Patients with repairable cardiac defects can receive cardiac repair and lung transplantation alone, and our experience includes children with atrial septal defect, ventricular septal defect (VSD), patent ductus arteriosus, vascular rings, atrioventricular canal defects, pulmonary vein stenosis, peripheral pulmonary arterial stenosis, and pulmonary atresia with non-confluent pulmonary arteries in addition to children with anomalies of pulmonary venous return.
An additional consideration in pediatric patients is the preference of single-lung versus bilateral sequential lung transplantation. In children with cystic fibrosis, bilateral lung transplantation is preferred to remove the infected lungs and decrease
the sources of potential sepsis. A similar consideration is given to patients with chronic bronchiectasis who may be best served by bilateral sequential transplantation and removal of infection sources in the lungs. Single-lung transplantation is a possibility in patients with pulmonary fibrosis and pulmonary vascular disease. Although there have been successful series of single-lung transplantations for primary and secondary pulmonary hypertension, these reports have suggested that the postoperative course is more complicated in such patients. The entire cardiac output is delivered to the transplanted lung if singlelung transplantation is used in the presence of severe pulmonary vascular disease. Thus, patients may be unstable in the postoperative period and have additional instability when infection, rejection, or bronchiolitis obliterans occurs in the transplanted lung. We, therefore, prefer bilateral sequential transplantation in most children with pulmonary vascular diseases to improve postoperative stability and the distribution of pulmonary blood flow. In addition, the use of bilateral sequential transplantation in younger children and infants allows for maximum possible growth and development of the lungs. Single-lung transplantation is still considered in children with primary pulmonary hypertension in whom there is a relative contraindication to entering one pleural space, such as those children who have had multiple previous thoracotomies with cyanosis.
the sources of potential sepsis. A similar consideration is given to patients with chronic bronchiectasis who may be best served by bilateral sequential transplantation and removal of infection sources in the lungs. Single-lung transplantation is a possibility in patients with pulmonary fibrosis and pulmonary vascular disease. Although there have been successful series of single-lung transplantations for primary and secondary pulmonary hypertension, these reports have suggested that the postoperative course is more complicated in such patients. The entire cardiac output is delivered to the transplanted lung if singlelung transplantation is used in the presence of severe pulmonary vascular disease. Thus, patients may be unstable in the postoperative period and have additional instability when infection, rejection, or bronchiolitis obliterans occurs in the transplanted lung. We, therefore, prefer bilateral sequential transplantation in most children with pulmonary vascular diseases to improve postoperative stability and the distribution of pulmonary blood flow. In addition, the use of bilateral sequential transplantation in younger children and infants allows for maximum possible growth and development of the lungs. Single-lung transplantation is still considered in children with primary pulmonary hypertension in whom there is a relative contraindication to entering one pleural space, such as those children who have had multiple previous thoracotomies with cyanosis.
DONOR SELECTION
Donors for cardiopulmonary or pulmonary transplantation are sparse compared with donors for other organs. Only 10% to 15% of cardiac donors may be suitable or donation of heart-lung blocks or lungs. This rarity reflects the damage often done to the lungs during gastric aspiration either at trauma or with a sudden neurologic event. In addition, severe pulmonary edema from either neurogenic or cardiac cause can affect oxygen exchange of the potential donor lungs. General criteria for pulmonary donors includes blood type compatibility, normal gas exchange with an arterial partial pressure of oxygen (PaO2) of >300 mmHg on 100% oxygen, and 5 cm H2O positive endexpiratory pressure. A clear chest X-ray film showing no infiltrates or contusions, age younger than 50 years with no history of pulmonary disease and a <20 pack-year smoking history, and normal findings on electrocardiograms and echocardiograms are criteria for cardiopulmonary and pulmonary donors. Bronchoscopy should confirm normal airway anatomy and easily cleared secretions with no ready accumulation of further secretions. A history of pulmonary disease or prolonged smoking or asthma is a relative contraindication for donation. In addition, demonstrated aspiration of gastric contents, contamination of the tracheobronchial tree, or severe lung contusion is considered contraindication to donor use. In some cardiopulmonary donors, the requirement of very high-dose inotropic drugs in the face of suitable fluid management or significant ventricular hypertrophy or dysfunction on an echocardiogram is considered a contraindication to using the combined heart-lung block.
As in all organ donation, the presence of human immunodeficiency virus or hepatitis A or B is a contraindication for use of donor organs. Hepatitis C organs may be used in hepatitis C positive recipient and in some cases may be used if the severity of the condition of the recipient warrants the risk of hepatitis C transmission. Although it is generally advisable to match CMV-seropositive donors with CMV-positive recipients, successful transplantation is not precluded by CMV mismatching. CMV prophylaxis is routinely used after transplantation and CMV infection is usually adequately treated if it occurs. Occasionally lungs that initially are deemed inappropriate for transplant may, with aggressive donor management, be rendered usable. Careful evaluation of each potential donor is therefore important to maximize the availability of suitable donor organs. Size matching between donor and recipient is important in pediatric lung and heart-lung transplantation. For heart-lung transplantation, it is desirable to have the donor weight within 20% to 30% of the weight of the recipient. Although it is possible in cardiac transplantation to use hearts of donors several times the body weight of the recipient, the fact that excessive lung size may compress the heart limits the size discrepancy that is acceptable for combined heart-lung transplantation in children. Donor-recipient size matching is more liberal when double-lung or singlelung transplant is contemplated. Because the lungs have the capacity to expand to fill chest cavities of significantly larger recipients, it is possible to use smaller donor lungs. In addition, it is possible to use large donor lungs and use lobes or trim portions of the parenchyma on the lungs to allow them to fill the chest cavity without impinging on cardiac function. Bronchial size match between donor and recipient is better correlated with height and age than with weight. Thus, most patients are listed for lung transplantation with a size range of 3 to 4 inches above and below the size of the recipient. However, heights of twice the size of the recipient can be considered if lobes are to be used from larger donors.
ORGAN PROCUREMENT
Flexible bronchoscopy is preferable at the time of lung procurement. The presence of direct trauma to the lungs or pulmonary contusions should be evaluated by direct inspection before the heart-lung or lung block is removed. The donor receives methylprednisolone and antibiotics and is heparinized before organ procurement. The technique of organ procurement from pediatric donors is similar to that of adult donors except that the volumes of cardioplegic and pulmonoplegic solutions are adjusted for the weight of the donor. Cardioplegic solution is given for a total dose of 30 cm3/kg of donor weight and pulmonoplegia for 50 cm3/kg donor weight. Antegrade crystalloid cardioplegic solution and Perfadex (extracellular low potassium dextran) pulmonoplegic solution has been used in most centers for organ preservation. Prostaglandin E1 (500 µg) is injected directly into the main pulmonary artery at the time of cross-clamp. Lungs are topically cooled with ice slush solution with both pleural spaces widely opened.
When a heart-lung block is harvested for a single recipient, the cardioplegic solution is administered into the aorta and pulmonoplegic solution directly into the pulmonary artery with venting of the heart by division of the left atrial appendage. Division of the inferior vena cava at the diaphragm permits evacuation of cardioplegia without distention of the ventricle. The trachea is mobilized above the level of the carina and minimal dissection of the carina and lateral trachea is done. The lungs are then gently inflated to a pressure of approximately 20 mmHg and the trachea stapled. The superior vena cava is ligated and divided and the esophagus mobilized in the superior mediastinum and stapled and divided. The aorta is transected at the level of the innominate artery, and the distal aorta in the posterior pericardial space is mobilized and ligated and also divided. If additional aortic length is necessary, the aorta is not transected, but the arch vessels are divided individually. With incision of the pleura at the paraspinal region, bilaterally, the entire heart-lung block is then excised and the esophagus then removed from the specimen. If the heart and lung are to be harvested separately, cardioplegic and pulmonoplegic solutions are administered as in the combined heart-lung
technique; however, the interatrial groove is developed and then the heart is eased by the division of the aorta and pulmonary artery, leaving the bifurcation of the pulmonary artery for the lung. The left atrium is then excised with a limited left atrial cuff, leaving as much as possible of the pulmonary venous confluence bilaterally for the lung implantation. The superior and inferior venae cavae are divided at the pericardial reflection. Many choose to use retrograde flush for the lungs at a dose of 5 to 10 ml/kg per pulmonary vein. The lung block is then excised as is the heart-lung block by the division of the trachea with gentle inflation of the lungs. The organs are then put in iced saline solution in sterile bags and transported to the recipient center on ice. Although some groups have used cardiopulmonary bypass for cooling of the entire heart-lung block, this technique is not widely used in the United States. Preservation times of >9 hours for lung transplantation have been successfully achieved with the use of these techniques.
technique; however, the interatrial groove is developed and then the heart is eased by the division of the aorta and pulmonary artery, leaving the bifurcation of the pulmonary artery for the lung. The left atrium is then excised with a limited left atrial cuff, leaving as much as possible of the pulmonary venous confluence bilaterally for the lung implantation. The superior and inferior venae cavae are divided at the pericardial reflection. Many choose to use retrograde flush for the lungs at a dose of 5 to 10 ml/kg per pulmonary vein. The lung block is then excised as is the heart-lung block by the division of the trachea with gentle inflation of the lungs. The organs are then put in iced saline solution in sterile bags and transported to the recipient center on ice. Although some groups have used cardiopulmonary bypass for cooling of the entire heart-lung block, this technique is not widely used in the United States. Preservation times of >9 hours for lung transplantation have been successfully achieved with the use of these techniques.
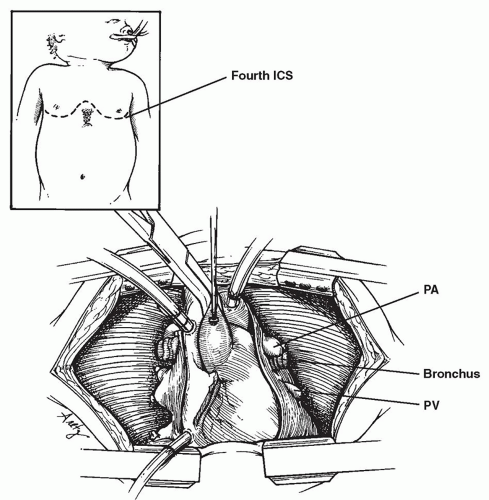
RECIPIENT OPERATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree