Healthcare-Acquired Pneumonia, Including Ventilator-Associated Pneumonia
INTRODUCTION
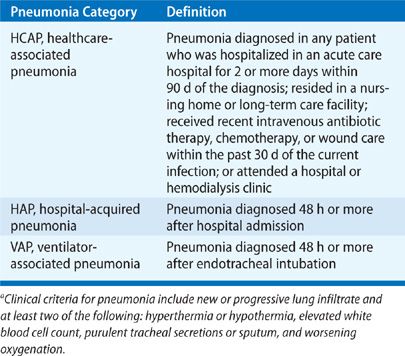
Healthcare-associated infections (HAIs) impose a significant economic and clinical burden on healthcare systems. The burden is magnified by increasing infection rates due to multi-drug resistant (MDR) pathogens. Nosocomial pneumonia remains an important etiology of HAIs and consists of three distinct entities: healthcare-associated pneumonia (HCAP), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP).1 Definitions for each of these conditions can be seen in Table 129-1. Three device-associated HAIs are reported to the National Safety Healthcare Network (NHSN), the surveillance branch of the Centers for Disease Control and Prevention (CDC). These include central line–associated blood stream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), and VAP. According to an annual summary by the NHSN published in 2008, VAP accounted for 15.9% of all the reported HAIs, placing third among device-associated HAIs.2 Nosocomial pneumonia results in excess healthcare utilization and leads to greater mortality. This chapter focuses on the microorganisms responsible for infection, the complexities surrounding the diagnosis, and the preventive and therapeutic management strategies used to combat nosocomial pneumonia.
EPIDEMIOLOGY
HAP, although not a reportable infection, is thought to occur at a rate of 5 to 10 cases per 1000 hospital admissions, and numerous studies indicate that VAP occurs in 9% to 27% of all intubated patients.1 In a 1-day prevalence study involving critically ill patients across Western Europe, pneumonia was the most common ICU-acquired infection occurring in 10% of patients and accounting for 47% of all ICU-acquired infections.3 An additional large study conducted in Europe revealed a similar pneumonia rate of 9%.4 These two studies, in addition to others,5 demonstrated that mechanical ventilation served as the most important risk factor for nosocomial pneumonia. Therefore, the CDC now reports the incidence of nosocomial pneumonia as cases per 1000 ventilator days.
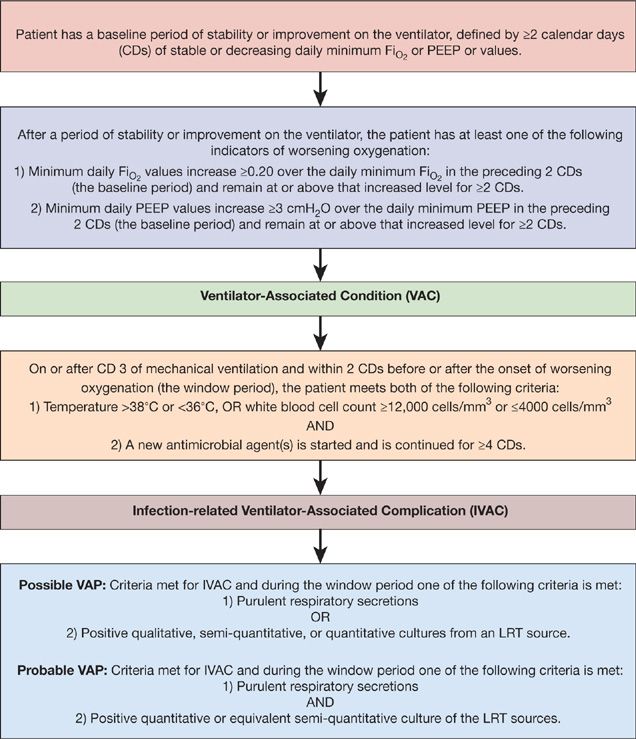
The NHSN functions as the CDC’s surveillance system for HAIs and collects data from more than 5000 participating healthcare facilities. The NHSN data summary report from 2010 categorized rates of VAP based on ICU location.6 Rates of VAP were highest in burn, surgical, trauma, and neurological/neurosurgical ICUs ranging from 2.5 to 6 cases per 1000 ventilator days. VAP was diagnosed less frequently in medical and medical/surgical ICUs occurring at a rate of 1 to 1.8 cases per 1000 ventilator days (Table 129-2). Recently, the validity of the surveillance system utilized by the NHSN to identify VAP has been questioned. The CDC/NHSN pneumonia (PNEU) definitions (Table 129-3) were thought to lack accuracy, objectivity, and consistency. As a result, the NHSN implemented a new surveillance algorithm for identifying ventilator-associated events (VAEs) in January 2013. The goal of the new algorithm is to identify complications of mechanical ventilation including, but not limited to, to VAP by using objective changes in ventilator parameters indicative of worsening oxygenation (Fig. 129-1).
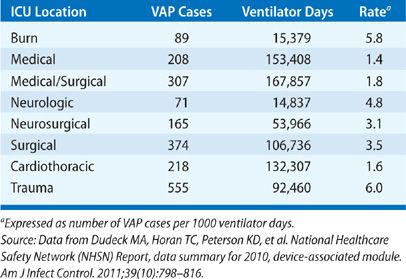
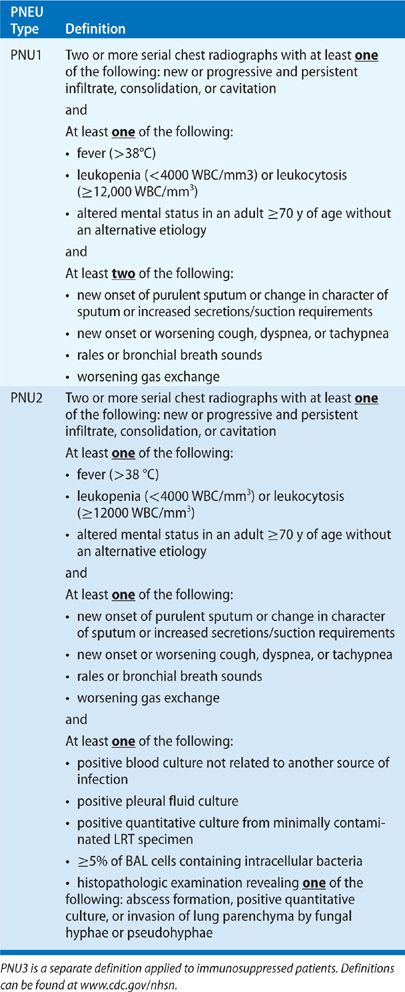
Figure 129-1 Abbreviated NHSN surveillance algorithm for ventilator-associated events.
VAP risk is highest early in the course of hospitalization and increases over time. A multicenter prospective cohort analysis conducted in Canada demonstrated a VAP rate of 14.8 cases per 1000 ventilator days, half occurring on or before day 7.7 While the cumulative risk for developing VAP increased with duration of ICU stay, the daily risk increased until day 5 and then subsequently decreased. Early-onset VAP usually confers a better prognosis as culprit organisms are more sensitive to antibiotics as opposed to the MDR pathogens often responsible for late-onset VAP (see also Chapter 125).1
Determining the economic impact of VAP and the associated morbidity and mortality has been difficult. Critically ill patients have multiple, evolving, complex disease processes making it difficult to isolate VAP as the sole cause of excess cost, morbidity, or death. Multiple studies have been performed with variable results. A case-control analysis revealed a crude mortality of 54%, an attributable mortality of 27%, and a 13-day increase in ICU length of stay compared to matched controls.8 The effect of VAP on clinical outcomes was slightly reduced in a larger, case-matched, prospective cohort study that demonstrated an attributable ICU length of stay of 4 days and an attributable mortality of 6%.9 Other case-matched trials failed to show any difference in mortality. A retrospective, matched cohort study using a large US database estimated an increase of >$40000 in mean hospital charges per patient.10 In addition, a more recent retrospective, matched cohort study using the ICD-9 code to identify VAP revealed a mean hospitalization costs of $99598, $39828 greater than those patients without VAP.11 Recent advances in statistical methodology allow for the control of the effect that severity of illness can have on the attributable mortality associated with VAP, not only at the time of infection but over the course of a hospitalization during disease evolution. Employing a statistical technique from the field of causal inference, an analysis of a large French national database resulted in a milder estimation of attributable mortality associated with VAP, 1% on day 30 and 1.5% on day 60.12 Additional factors, particularly the timely initiation of appropriate antibiotics, certainly affect the aforementioned estimates. While debate continues over the attributable mortality of VAP, the condition imposes a significant clinical and economic burden.
MICROBIOLOGY
Etiologic agents for nosocomial pneumonia depend on multiple factors including underlying disease, geography, ICU population, duration of mechanical ventilation, previous antibiotic therapy, and the method used to obtain respiratory cultures. Historically, aerobic gram-negative bacilli (GNB) represent the most prevalent pathogens causing nosocomial pneumonia. Enterobacteriaceae embody an important group of bacteria given the importance of aerodigestive colonization in the pathogenesis of VAP. Microorganisms within this group include Klebsiella, Escherichia coli, Enterobacter, Citrobacter, Proteus, and Serratia species. Additional GNB include Pseudomonas, Acinetobacter, and Stenotrophmonas species. These bacteria are ubiquitous in the environment and have minimal nutritional requirements making them suitable for the colonization of hospitalized patients. Summarizing VAP data from 24 trials, GNB represented 58% of recovered organisms.5 Pseudomonas aeruginosa was the most prevalent pathogen accounting for 24% of cases. According to a more recent report by the NHSN in 2008, at least 47% of cases of VAP reported in 2006 and 2007 were due to GNB (Table 129-4).2
TABLE 129-4 Distribution and Rank of 5960 Isolated Pathogens Associated with VAP Reported to the NHSN between January 2006 and October 2007
With the emergence of MDR pathogens, gram-positive organisms, mainly methicillin-resistant Staphylococcus aureus (MRSA), have become an increasingly important cause of VAP. A review of the European Prevalence of Infection in Intensive Care study revealed that 31% of nosocomial pneumonias were due to S. aureus,3 and the previously mentioned summary of 24 trials determined that S. aureus was responsible for 20.4% of cases of VAP.5 In the NHSN report from 2008, S. aureus accounted for 24% of reported cases and was the most common etiologic agent, greater than P. aeruginosa (16%).2
Bacteria responsible for community-acquired pneumonia such as Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae can cause VAP but occur less frequently and often earlier in an episode of mechanical ventilation. Anaerobes, fungi, and Legionella species are uncommon causes of VAP but cases have been reported. Also, multiple microorganisms can infect a host leading to polymicrobial infection.
As mentioned earlier, multiple factors can influence the specific microorganism responsible for VAP, but the most important and well studied are the duration of mechanical ventilation at the time of diagnosis, prior antimicrobial use, and local hospital infection patterns. An investigation into the role of stress ulcer prophylaxis on the development of nosocomial pneumonia demonstrated that common organisms such as S. pneumoniae and H. influenzae along with S. aureus were responsible for the majority of cases of early-onset pneumonia.13 In contrast, GNB other than H. influenzae accounted for most cases of late-onset pneumonia. A multivariate analysis conducted in France determined that duration of mechanical ventilation and prior broad-spectrum antibiotic use were significant risk factors for VAP due to potentially drug-resistant bacteria such as MRSA, P. aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia.14 Furthermore, a Spanish retrospective review proved that previous antibiotic use resulted in higher rates of P. aeruginosa and increased mortality likely due to drug resistance.15 The variability of microorganisms isolated in cases of VAP among four different centers in Spain and France despite similar patient characteristics highlighted the importance of recognizing a center’s local infection profile.16
PATHOPHYSIOLOGY
Nosocomial pneumonia most often occurs through the aspiration of microorganisms colonizing the aerodigestive tract in a host with altered immunity. Direct inhalation or hematogenous dissemination of bacteria is less frequent route of infection. Aspiration during sleep is present in a large number of normal subjects, but the volume aspirated is small and more importantly the pathogenicity of the microorganisms is diminished. Hospitalized patients are considered more likely to aspirate larger volumes of virulent bacteria with greater frequency due to decreased sensorium, difficulty swallowing, and impaired gut motility.
The role of colonization of the upper respiratory and digestive tracts has been extensively evaluated. Previous studies demonstrated that 23% of patients admitted to a medical ICU colonized with GNB developed a nosocomial respiratory infection compared to only 3% of noncolonized patients.17 Later, genomic DNA analysis proved that strains of bacteria isolated from the lower respiratory tract (LRT) in cases of nosocomial pneumonia were identical to those strains colonizing the oropharynx and/or stomach.18 Hospitalized patients are at risk for colonization due to the potential for contaminated respiratory equipment and hospital water systems, the transmission of bacteria via healthcare workers, the spread of microorganisms through respiratory droplets, and pharmacologically induced alterations in gastric pH.
The endotracheal tube serves as a reservoir for bacteria from the upper airway to leak around the cuff and gain entry into the LRT. Manufacturers have developed novel endotracheal tubes with antimicrobial properties that prevent biofilm formation.19 Humidifiers and nebulizers can be infected allowing for the transfer of microorganisms between patients by respiratory personnel. Contaminated water supply systems have led to previous outbreaks of nosocomial Legionella species. Fungal infections have resulted from hospital renovations and construction, contaminated air supply, and poor filtration.
Critically ill patients requiring mechanical ventilation are prone to develop stress ulcers and thus are often treated with histamine Type 2 (H2) antagonists and proton pump inhibitors for prevention.20 In addition, patients frequently require enteral nutrition. Although these strategies provide effective prophylaxis and nutrition, alterations in gastric pH promotes growth of bacteria in a normally sterile environment potentially increasing the risk for nosocomial pneumonia.13,21
The early, hyperinflammatory response that occurs in trauma and sepsis can cause multisystem organ failure and death. Improvements in the management of sepsis have decreased rates of early death and patients now often succumb to protracted or nosocomial infections. Recent evidence suggests that a subset of patients who survive the cytokine storm associated with the proinflammatory response of sepsis develop an immunodeficient state promoting viral reactivation and nosocomial infection including pneumonia. Immunomodulator therapies are currently being investigated.21
RISK FACTORS
Given the important pathophysiologic roles of aspiration and colonization of the aerodigestive tract, it is logical that the significant risk factors for nosocomial pneumonia involve these processes. A complete list of risk factors can be visualized in Table 129-5.
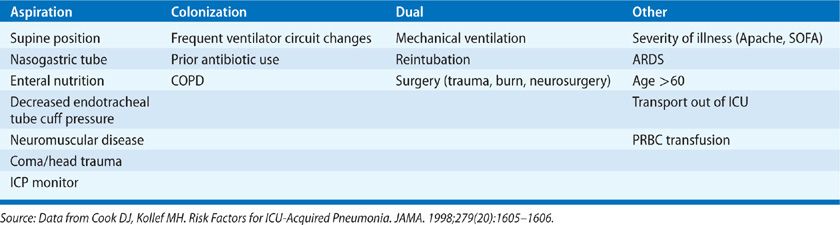
Mechanical ventilation is the single most important risk factor for nosocomial pneumonia increasing the risk up to 20-fold.1,4,22 Reintubation also has been associated with the development of VAP.23 Condensate within the ventilator tubing can become colonized with bacteria and lead to infection if introduced into the LRT. A 2012 Cochrane review found that heat-moisture exchangers that collect less moisture may reduce the incidence of VAP when compared to heated humidification.24 There is no evidence that frequent changes of the ventilator circuit reduce VAP rates,25,26 nor does early tracheotomy.27
Severity and type of disease are known risk factors for nosocomial PNA. An Apache II score ≥16 and an organ system failure index of 3 or greater have both been linked to VAP.4,28 Nosocomial PNA occurs more frequently in postsurgical patients, particularly burn and trauma victims.6,29 Incidence is also higher in COPD likely due to greater colonization rates and ARDS as a result of prolonged mechanical ventilation.30–32
Aspiration occurs more frequently in the supine position and can be exacerbated by the presence of a nasogastric tube combined with early enteral nutrition.33–36 Postpyloric feeding may reduce gastroesophageal reflux but the impact on VAP incidence is uncertain. Antacids and histamine Type 2 (H2) antagonists used for stress ulcer prophylaxis have proved to be independent risk factors for nosocomial PNA as alterations in gastric pH promote colonization.37,38 In addition, prior antibiotic administration can select for infection by MDR microorganisms.14,28
DIAGNOSIS
The diagnosis of nosocomial pneumonia has traditionally been difficult. It should be suspected in any patient with a new or progressive radiographic infiltrate and signs or symptoms indicative of infection such as fever, leukocytosis or leukopenia, purulent sputum, and worsening oxygenation. The accuracy of using the aforementioned clinical findings in diagnosing nosocomial pneumonia ranges depending on the number and type of clinical criteria and is complicated by the lack of a gold standard reference. Even postmortem histologic examination is not 100% specific. The addition of microbiological data to clinical criteria has not definitively improved diagnostic accuracy. The problems associated with diagnosing VAP produced challenges for surveillance systems such as the NHSN. Therefore, as mentioned previously, the CDC created a new surveillance algorithm for the identification of VAEs that include both infectious and noninfectious complications of mechanical ventilation (Fig. 129-1). Due to the lack of a proven diagnostic method, two different strategies have been used and compared using clinical or bacteriologic criteria, each associated with advantages and disadvantages.1
The clinical strategy employs the abovementioned clinical and radiographic criteria in diagnosing nosocomial pneumonia. A combination of two out of three clinical criteria and a radiographic infiltrate yielded a sensitivity of 69% and a specificity of 75% for the diagnosis of VAP in 25 mechanically ventilated patients using histology and quantitative lung tissue culture on autopsy as the reference. Increasing the number of clinical criteria resulted in greater specificity but at the cost of lesser sensitivity.39 However, in a postmortem analysis of 39 mechanically ventilated patients, clinical criteria did not provide a reliable positive predicted value for histologic pneumonia.40 A semiquantitative endotracheal aspirate culture can be used to identify a pathogen and, if positive, has been shown to correlate with quantitative cultures of the LRT obtained via protected specimen brush (PSB).41 Also, a negative endotracheal aspirate culture has high sensitivity and good negative predictive value if antibiotics have not recently been changed.42 However, semiquantitative cultures are not as reliable as quantitative cultures of the LRT due to an inability to differentiate between colonization and infection. The use of clinical criteria and a reliance on semiquantitative cultures can be overly sensitive resulting in unnecessary antibiotic use and potentially delay the identification of extrapulmonary infection.
The bacteriologic strategy uses quantitative cultures of the LRT via endotracheal aspirate, PSB, or bronchoalveolar lavage (BAL) to confirm or eliminate the diagnosis of nosocomial pneumonia based on thresholds of bacterial growth. These thresholds differ depending on the method of respiratory sampling and are as follows: ≥105 colony-forming units (CFU)/mL for an endotracheal aspirate, ≥104 CFU/mL for a BAL, and ≥103 CFU/mL for a PSB. Results of these procedures guide decisions such as when to initiate or stop antibiotics and which drug should be used against the offending agent. There are no definitive data to support the use of one sampling technique over another; however, the cellular analysis of BAL fluid may provide an advantage as a sample containing <50% neutrophils produced excellent negative predictive value in one study.40 Also, given the multifocal nature of VAP, even mini-BAL samples obtained blindly without the use of bronchoscopy can be effective.43–45 The bacteriologic strategy has resulted in less and more narrowed antibiotic use, an important point given the surge of antibiotic-resistant microorganisms in the ICU setting.46–48 A major disadvantage with this approach is the concern for false negatives, which could result in cases of nosocomial pneumonia going untreated, especially in the setting of recently introduced antibiotics.49
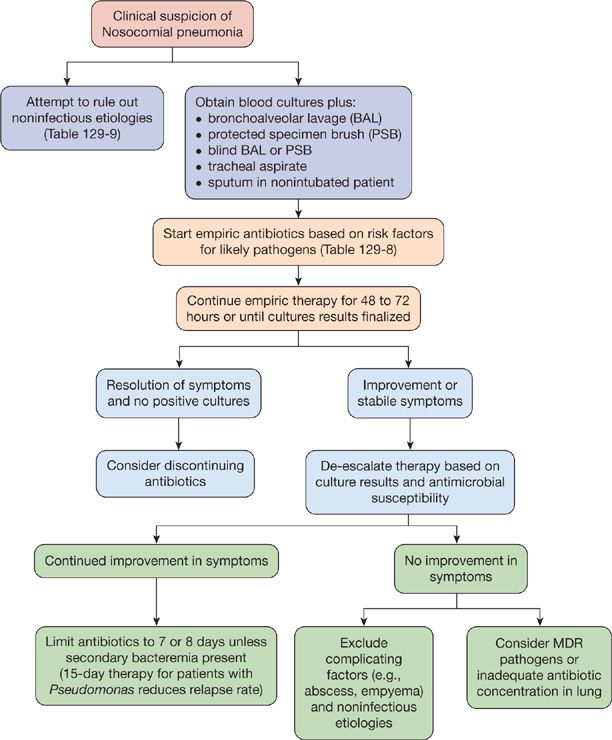
Multiple studies have compared the clinical and bacteriologic strategies. Only one prospective, randomized trial demonstrated a mortality benefit at 14 days when using the bacteriologic strategy.46 Others have failed to reproduce these findings, including a large study conducted by the Canadian Critical Care Trials Group and a comprehensive meta-analysis.50,51 In addition, the bacteriologic strategy does not seem to reduce the duration of mechanical ventilation or ICU length of stay. Ultimately, the decision to employ either strategy rests with the clinician on a case-by-case basis. If bronchoscopic sampling can be performed safely and the appropriate personnel is available, it is reasonable to utilize this approach as antibiotic decisions may change based on culture results. If the clinical strategy is used, the clinician should reevaluate the patient often for guidance on antibiotic usage. Regardless of the diagnostic strategy, an unstable patient with a high pretest probability of nosocomial pneumonia should be initiated on empiric antibiotics as a delay in antibiotic administration leads to higher mortality.52–57 A diagnostic algorithm adapted from the Washington Manual of Critical Care Medicine can be seen in Figure 129-2.
Figure 129-2 A step-by-step approach to the management of nosocomial pneumonia. (Reproduced with permission from Kollef M, Isakow WA. The Washington Manual of Critical Care, 2nd edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.)
PREVENTION
Prevention of nosocomial pneumonia centers on minimizing mechanical ventilation and reducing aspiration and bacterial colonization. As mentioned previously, mechanical ventilation is the most important risk factor for nosocomial pneumonia.1,4,22 Therefore, avoiding intubation and reintubation, shortening the duration of mechanical ventilation, and employing noninvasive methods to improve oxygenation and ventilation when indicated can prevent pneumonia.22,23,58
Multiple methods designed to reduce bacterial colonization of the aerodigestive tract have been evaluated for their impact on VAP incidence. Selective decontamination of the digestive tract (SDD) administers nonabsorbable antimicrobial agents directly to the oropharynx and stomach to prevent colonization with GNB and S. aureus. While SDD has been shown to have a beneficial effect on nosocomial infection rates and even survival, potential negative effects include cost and the selection for antibiotic-resistant microorganisms when utilized in ICUs with high rates of endemic antibiotic resistance.59–62 Alternatively, selective oropharyngeal decontamination (SOD) with nonabsorbable antibiotics or chlorhexidine provides a cheaper approach with similar efficacy.62,63 While systemic antibiotics given once at the time of intubation may reduce early-onset VAP, the routine use of prolonged prophylactic, systemic antibiotics cannot be recommended.64,65 In addition, probiotics may reduce VAP rates but no studies to date demonstrate a survival benefit.66
Strategies to reduce aspiration have also been studied. Patients should not be managed in a supine position, but rather placed in a semi-recumbent position.67 Continuous aspiration of subglottic secretions has decreased the incidence of VAP in cardiac surgery patients.68,69 Also, postpyloric feeding may reduce aspiration but the effect on infection rates and mortality is unknown. In addition, there is no evidence favoring a specific time point for the initiation of enteral feeding.70
The Institute for Healthcare Improvement developed a programmatic bundle, used by many healthcare institutions in the United States, designed to improve the care of mechanically ventilated patients. Components addressing the prevention of VAP include positioning the patient with the head of the bed elevated at 45 degrees, daily sedation holidays coupled with an assessment of readiness to extubate, and daily oral care with chlorhexidine. Each component, when evaluated independently, has been associated with the prevention of VAP.71 A similar bundling strategy developed by French investigators was evaluated prospectively in a single medical ICU. Components of the bundle included hand hygiene, head of bed elevation, maintenance of tracheal cuff pressure, avoidance of gastric overdistention, good oral hygiene, and minimization of tracheal suction. After an initial educational program for healthcare personnel, compliance with each component increased throughout the 24-month long study.72 In a companion evaluation, total VAP rates decreased from 22.6 to 13.1 per 1000 ventilator days after implementation of the bundle.73 A multicenter trial conducted in Spain employing a bundle with comparable measures reduced VAP rates from 12.9 to 9.8 per 1000 ventilator days despite a compliance rate of less than 30% for all five measures.74 Thus, the bundling strategy appears to reduce VAP rates and could potentially produce a more substantial impact with improved compliance. Table 129-6 contains a list of recommended prevention strategies.